Continued
6. Nature’s New Discovery: Another Unidentified Anti-cancer Approach of PD-1 / PD-L1 Antibodies
doi: 10.1038/nature22396.
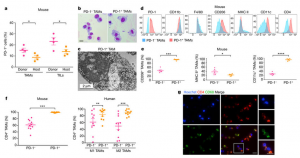
On May 17, Nature published an online article titled “PD-1 expression by tumor-associated macrophages inhibits phagocytosis and tumor immunity”, revealing the immune checkpoint inhibitors represented by the PD-1 / PD-L1 antibody can fight cancer in a completely different way.
Dr. Irving Weissman, a professor of pathology and developmental biology at Stanford University School of Medicine, led the team to find that in addition to mobilizing T cells, these antibodies can “unblock” tumor-associated macrophages (TAMs) to “engulf” cancer.
Tumor-associated macrophages are macrophages infiltrated in tumor tissue and are the most abundant immune cells in the tumor microenvironment. In the early stages of tumor development, these cells are responsible for identifying and removing tumor cells. However, with the development of tumors, macrophages will help spread and metastasize cancerous cells.
The team found that the PD-1 receptor also inhibits the anticancer activity of tumor-associated macrophages. “The same type of macrophages express PD-1 receptors on the surface, and when PD-1 or PD-L1 is blocked by antibodies, TAMs, whose inhibition then be removed, restores phagocytosis and elimination of cancer cells,” Weissman explains.
The researchers hope this new finding will improve and expand the efficiency and use of immunotherapy.
7. Science: Tumor MMR Gene Defects Predict Therapeutic Effect Of PD-1 Inhibitors!
DOI:10.1126/science.aan6733
Until recently, PD-1 inhibitors were only approved for the treatment of a small number of cancers, such as rectal cancer. However, a recent clinical study found that certain tumor-specific gene defects may serve as clinical markers for predicting the degree of response of cancer patients to immunotherapy with PD-1 inhibitors.
The authors explain that because clinical tests of these genetic defects are so easy to perform, thus these genetic defects can serve as a new standard in predicting the extent to which tumors respond to immunotherapy and can help physicians find patients who react to immunotherapies in a more effective way. This clinical trial contains 86 patients with 12 different cancers. Dung Le and so on found that the immunotherapeutic Pemetrexed (anti-PD-1 antibody) is effective in a variety of cancers.
The Pemetrexed treatment successful controlled the progression of disease in 66 patients, and tumor even completely disappeared in 18 patients. They find these genomic repair pathways, including mismatch repair (MMR) in these responding tumor tissues, all have genetic defects. PD-1 inhibitors can enhance the immune system to attack a variety of malignant tumor cell surface abnormal protein called tumor antigen, these proteins are generated and enriched in the tumor cell surface due to the tumor inherent instability of the genome.

8. Science: A new breakthrough in cancer immunotherapy! PD-1 blockage therapy-activated T cells also depend on CD28 co-stimulation
doi:10.1126/science.aaf0683
Anti-cancer drugs (also called immune checkpoint inhibitors) that block the PD-1 pathway are now approved by the U.S. Food and Drug Administration (FDA) to treat melanoma, lung cancer, and several other cancers. These drugs are often described as “releasing the brakes” on the surface of dysfunctional T cells.
In a new study, researchers from the Emory vaccine center at the Emory University School of Medicine and the Winship Cancer Institute confirmed that even with the release of brakes impressed on PD-1, these tumors specific T cells still require “fuel” to proliferate and restore a potent immune response. This fuel comes from a CD28 molecule based co-stimulation. Relevant findings were published online March 9, 2017, in the journal Science, entitled “Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent.”
Despite the success of PD-1 targeting drugs, many patients’ tumors do not respond to them. The findings of this study suggest that CD28 present on the surface of T cells may be a clinical biomarker that predicts the effectiveness of PD-1 targeting drugs. In addition, the demand for CD28 suggests that co-stimulation maybe lost in some patients, which may help guide the design of combination therapies.
9. Combined: PD-1 / PD-L1 inhibitors enhance the efficacy of Imatinib in the treatment of gastrointestinal cancer
DOI:10.1158/1078-0432
Tyrosine kinase inhibitors are usually effective for gastrointestinal stromal tumors (GISTs) but are often ineffective because they develop resistance. Immunotherapy, especially PD-1 or PD-L1 inhibitors, has shown an astonishing effect in a number of cancer treatments. But so far the efficacy of PD-1 / PD-L1 inhibitors on GISTs is still blank.
Based on this, researchers from the Memorial Sloan Kettering Cancer Center analyzed tumor and blood samples from 85 patients with GISTs and analyzed the expression of immunosuppressive molecules by flow cytometry. Finally, they found that PD-1 / PD-L1 inhibitors combined with Imatinib demonstrated therapeutic efficacy in mice immunized with KitV558Δ/+ and the results were published in Clinical Cancer Research recently.
They found that PD-1, lymphocyte activation gene 3, T-cell immunoglobulin mucin 3 were highly expressed on the surface of tumor-infiltrating T lymphocytes compared to blood T lymphocytes, whereas T-cells in patients treated with PD-1 / PD-L1 inhibitors and Imatinib combined had the highest PD-1 expression. Meanwhile, PD-L1 expression levels in tumor-infiltrating T lymphocytes are quite different. Treatment of human GIST cell line with Imatinib abolished PD-L1 up-regulation induced by IFNγ by inhibiting STAT1. In KitV558Δ/+ mice, Imatinib can downregulate the expression of IFNγ-related genes and PD-L1 in tumor cells. Although PD-1 / PD-L1 alone has no effect on GISTs, it can enhance the tumor-killing function of T cells under the inhibition of KIT and IDO, thereby significantly enhance the anti-cancer effect of Imatinib.
10. New England Journal of Medicine: Detailed PD-1 signaling pathway
DOI:10.1056/NEJMra1514296
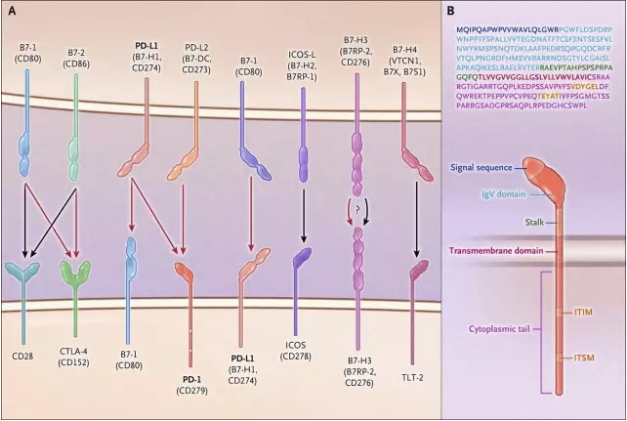
The signaling pathway consisting of the PD-1 receptor and its ligands PD-L1 and PD-L2 plays a crucial role in maintaining peripheral immune tolerance. Many tumors and pathogens can take advantage of this pathway to escape T cell-mediated immune responses to tumors and pathogens. Antibody therapies that target PD-1 and its ligands have been successful in many cancers. Along with antibodies targeting CTLA-4, these antibody therapies bring a revolution to the treatment of cancer. Recently, articles published in the New England Journal of Medicine (NEJM) specifically addressed the PD-1 signaling pathway.
PD-1 belongs to a member of a broad family of proteins, members of this family also include B7, CD28, CTLA-4 and other members. As shown in the figure below, some members of this family can stimulate T cell activation (black arrows represent stimulation signals) whereas CTLA-4, B7-1, and PD-1 proteins act as receptor mediators to suppress T cells active signal (red arrow represents inhibitory signal). The discovery of these immune checkpoints that suppress T-cell activation represents an important shift in the strategy for cancer immunotherapy. In the past researchers attempted to stimulate anti-cancer immune responses by boosting signaling, and today’s strategy has turned to an anti-cancer immune response that is enhanced by blocking signals that suppress T-cell activation.
Reference:
- Gordon SR et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017 May 25;545(7655):495-499. doi: 10.1038/nature22396. Epub 2017 May 17.
- Le DT et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017 Jul 28;357(6349):409-413. doi: 10.1126/science.aan6733. Epub 2017 Jun 8.
- Kamphorst AO et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017 Mar 31;355(6332):1423-1427. doi: 10.1126/science.aaf0683. Epub 2017 Mar 9.
- Seifert AM et al. PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors. Clin Cancer Res. 2017 Jan 15;23(2):454-465. doi: 10.1158/1078-0432.CCR-16-1163. Epub 2016 Jul 28.
- Vassiliki A. Boussiotis. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016; 375:1767-1778 November 3, 2016, DOI: 10.1056/NEJMra1514296