(Continued)
4. Nat Commun: Latest research finds the key protein mechanism, which regulates heart and muscle
David Giganti et al, Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity, Nature Communications (2018). DOI: 10.1038/s41467-017-02528-7
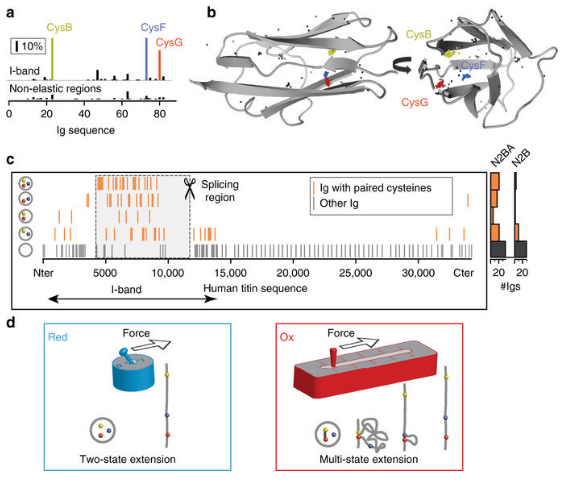
Scientists from CNIC and Columbia University have identified a key protein regulatory mechanism that regulates skeletal muscle and heart muscle function. Research results have recently been published in Nature Communications. The study was led by Professor Cristian Jorge Alegre-Cebollada, who described a new mechanism for regulating the elasticity of myosin. According to Professor Alegre-Cebollada, it is a key protein for the striated muscle working, especially in heart, “The evidence is that mutations in the musinin gene are common causes of diseases that affect the function of the body and heart muscle.”
Musinin is the largest protein in the human body and has many functions. According to Professor Alegre-Cebollada, “Simply speaking, we can compare the musinin to the molecular spring, which can make the muscle contract synchronously.” But it is not a simple spring, many mechanisms determine the elasticity of the muscle protein, including the folding of special structures of immunoglobulin domains. In summary, the elasticity of the musinin is determined by the domains of more than 100 immunoglobulins.
Using bioinformatics and structural biology, the team found that these immunoglobulin domains are rich in cysteines, which give them special properties. Jorge Alegre-Cebollada explained: “When two cysteines on a protein close to each other, a disulfide bond is formed between the two chains of the protein.” The researchers observed many disulfide bonds are formed in the immunoglobulin domains, and the cysteines involved in disulfide bond formation undergo dynamic changes, that is, isomerization.” The most interesting discovery is that the formation and isomerization of disulfide bonds can lead to dramatic changes in the elasticity of the protein.”
The formation of disulfide bonds belongs to one large class of biochemical transformations – redox. This process is known to cause many heart-related diseases, such as myocardial infarction.
Alegre-Cebollada’s team is currently investigating how human cells alter the reduced state of the musinin to regulate skeletal and cardiac muscle activity, and how different diseases interfere this process leading to function loss. Alegre-Cebollada added: “We discovered these phenomena during rebuilding the traction system in vitro. Although we learned a lot of new knowledge from this process, it is still difficult to understand how these basic principles can affect the living body. This is what we now focus on. This study combines physiology, biology, and biochemistry.”
This study is the result of collaboration between Dr. Alegre-Cebollada and Professor Julio Fernández of Columbia University. Professor Julio Fernández has been working on the development of single-molecule biophysical techniques to study the mechanical properties of proteins, and his collaboration with Alegre-Cebollada has made it possible to elucidate the biochemical regulatory mechanisms of the mechanical properties of proteins.
5. Cell Rep: New Technology Helps Uncover the Causes of Heart Disease
Cell Reports (2018). DOI: 10.1016/j.celrep.2017.12.045
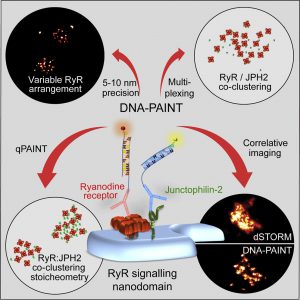
A recent study used new technologies to unravel the secrets of cell-to-cell communication: researchers from universities such as Cambridge University used a new method to make in-depth knowledge of a signal station called “nanodomain” inside cells.
They believe that this technology can help to understand the serious human diseases, such as heart disease, and provide potential treatment.
The Nanodomain is the key factor to drive the important physiological responses of the body, and it is also the cause of various diseases. So far, scientists often use electron microscopy to observe this structure, but this technology cannot reveal the molecular mechanism on how the nanodomain work.
Researchers from the United Kingdom have optimized an optical super-resolving fiber technology that enables high-quality imaging of this signaling site in human heart tissue. The results are published in Cell Reports.
The chief scientist of the study, Professor Christian Soeller, said: “None of us had thought that we could see a single nanodomain molecule through a light microscope more than ten years ago. This was due to the lack of previous super resolution technology. Afterward, with a series of the advancement of technology, we can finally use synthetic DNA to accomplish this goal.”
This optimized technology can help researchers observe any molecule in the cell, including its molecular characteristics and arrangement.
Based on the experimental results, the researchers believe that this new type of imaging technology can help us to understand more deeply on the principles of these molecular signal workstations and be able to design drugs that target more precisely.
Dr. Isuru Jayasinghe, the author of the study, stated: “At present, we do not have anti-heart failure drugs targeting this molecular structure, and the latter is the main cause for heart failure.”
6. JAHA: Be careful! Women have a greater risk of death after the heart attack!
Oras A Alabas et al. Sex Differences in Treatments, Relative Survival, and Excess Mortality Following Acute Myocardial Infarction: National Cohort Study Using the SWEDEHEART Registry, Journal of the American Heart Association (2017). DOI: 10.1161/JAHA.117.007123
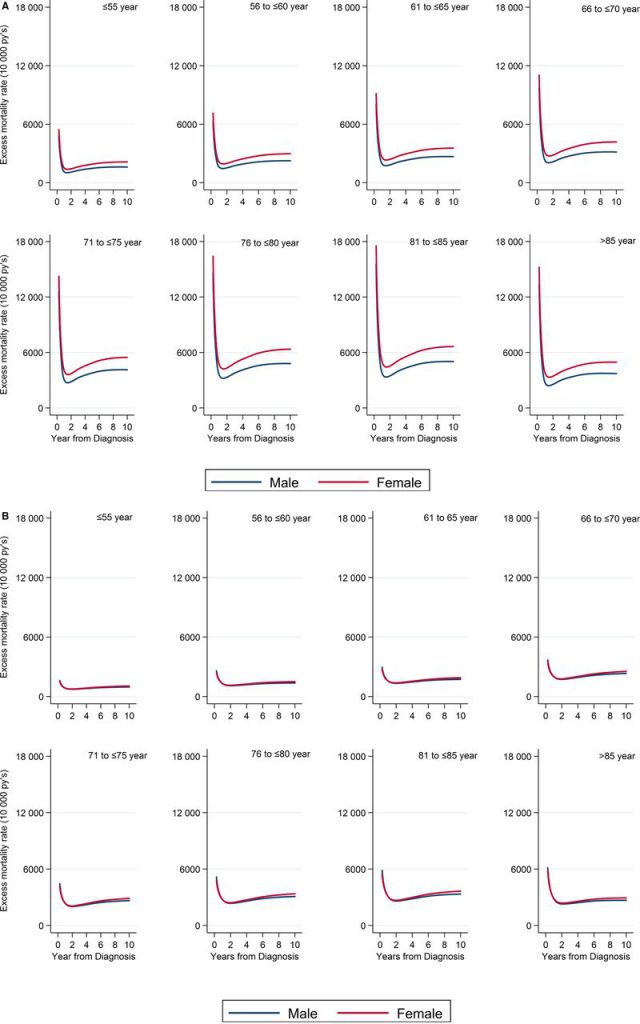
According to a new study, if British women are simply treated as men after a heart attack, fewer women will die each year.
Scientists from the University of Leeds in the UK and the Karolinska Institute in Sweden used data from the Swedish online cardiology registry (SWEDEHEART) to analyze the prognosis of 180,368 patients suffering from heart disease during the 10 years prior to December 2013.
By deducting the expected death from the population, the researchers found that the death rate of women within one year after the heart attack was even three times higher than that of men.
Although the data used in this study comes from Sweden, the researchers believe that the situation of British women may be worse than Sweden, because Sweden is one of the countries with the lowest incidence of heart disease in the world.
The study was recently published in the Journal of the American Heart Association and is jointly funded by the British Heart Foundation.
Chris Gale, the co-author and professor of cardiology at Leeds University, said: “We should try to change the misperception that heart disease affects only a specific group of people. Usually, when we mention a heart attack patient, we have an image of smoking obese middle-aged man with diabetes in our heads. But it is not always that case; heart disease affects a wide range of people, including women. Sweden is very prominent in medical care, the mortality rate of heart disease is the lowest in the world, but we still see there is a huge difference between the male and female’s treatment and the outcome of the disease. It is very likely that the situation of women in Britain is worse.”
Data analysis in Sweden found that the rate of women who suffered a coronary artery thrombosis due to a heart attack receiving the clear embolization treatment to restore blood flow (including heart bypass surgery and stents) is 34% lower than men.
More importantly, if women receive all the treatments recommended for the prognosis of heart disease, the difference in mortality due to heart disease between the sexes is significantly reduced.
Professor Gale added: “The results of this study show that we have a clear and simple way to improve the prognosis of women with heart disease. We need to ensure that women receive equal evidence-based care.”
Previous research by the British Heart Foundation has shown that women undergoing erroneous initial diagnosis are 50% more than men. At the same time, women receive less preoperative ECG, which is essential for rapid diagnosis and treatment.
Professor Jeremy Pearson, Deputy Director of Medicine at the British Heart Foundation, said: “Heart disease is usually considered a male health problem, but there are more women in the UK who died of coronary heart disease than breast cancer. The findings are worrisome – female patients with heart disease are dying for they have not received the correct treatment.”
7. eLife: Why can pregnant women’s diabetes induce neonatal congenital heart disease?
Haruko Nakano,Itsunari Minam,Daniel Braas,et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis, eLife (2017). DOI: 10.7554/eLife.29330
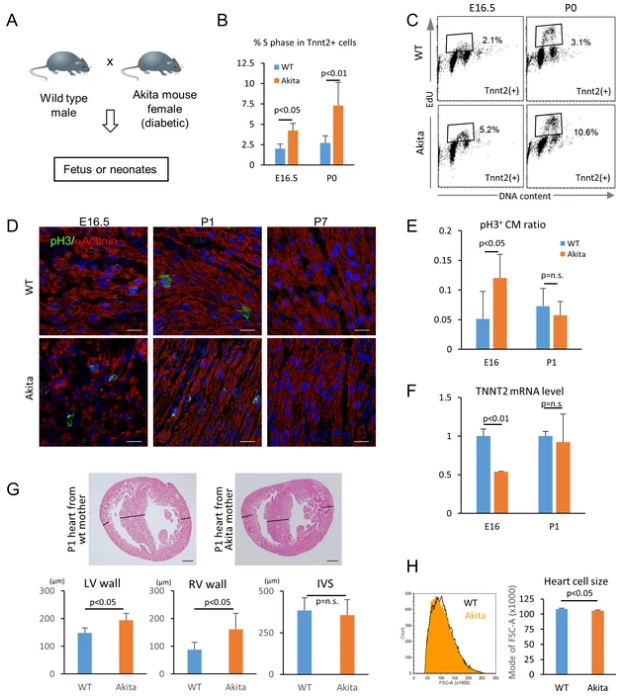
Recently, in a study published in the international magazine eLife, researchers from the University of California, Los Angeles (UCLA) found that high levels of blood glucose (irrespective of diabetes or other factors) may inhibit normal heart cells’ development. Related research can help explain why babies born from women with diabetes are more likely to have congenital heart disease.
When the developing heart cells are exposed to higher glucose levels, cells will produce more DNA building elements than normal, resulting in continuous cell reproduction rather than maturation; Nakano said that high blood glucose levels are not only unhealthy for adults but can also affect developing fetuses. The molecular mechanism by which hyperglycemia induces fetal illness may help develop new therapies for the effective treatment of diseases such as congenital heart disease.
Although genetic factors play a key role in the development of congenital heart disease, the main non-genetic risk factor for congenital heart disease is diabetes during pregnancy. The risk of congenital heart disease in infants whose mothers have higher blood sugar level is 2-5 times higher than others. However, researchers are currently not sure why glucose affects the developing fetus.
To this end, Nakano and his colleagues used human embryonic stem cells to grow cardiomyocytes in the laboratory and exposed them to different glucose levels. The results showed that cells exposed to lower glucose levels can develop normally, but cells exposed to higher glucose levels exhibited later maturation or complete immature performance, and these cells would later develop into more immature cells. The researchers found that when exposed to extra glucose, cardiomyocytes over-activate the pentose phosphate pathway, producing the basic components that make up the DNA. In the cells with higher glucose levels, the pentose phosphate pathway may make more nucleotides than normal, and excessive DNA basic elements will inhibit cell maturation.
The researchers said that excessive nutrients are considered to be very beneficial to cells, but in this study, they found that this is not actually the case. By eliminating glucose at the appropriate stage of development, researchers can effectively limit cell proliferation and promote it’s mature and make the heart muscle cells stronger. At present, the researchers also got the same conclusion in pregnant mice with diabetes. The heart cells of the mouse fetus can divide quickly but mature slowly.
At present, congenital heart disease in the United States affects approximately 1% of neonatal health. Congenital heart disease is the most common birth defect. The degree of illness in children is not the same, some children experience mild myocardial cell degeneration, and some have no symptoms or severe heart malformations. This study also showed that the pentose phosphate pathway may serve as a novel target to help researchers develop new methods that can effectively promote cardiac cell maturation. This study may help researchers to develop better ways to make cardiomyocytes from stem cells. Many methods that researchers have developed in the laboratory to produce cardiomyocytes can lead to the production of immature cells, but by inhibiting the pentose phosphate pathway may help produce more mature cells for heart cell regeneration.
Reference
- David Giganti et al, Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity, Nature Communications (2018). DOI: 10.1038/s41467-017-02528-7
- Cell Reports (2018). DOI: 10.1016/j.celrep.2017.12.045
- Oras A Alabas et al. Sex Differences in Treatments, Relative Survival, and Excess Mortality Following Acute Myocardial Infarction: National Cohort Study Using the SWEDEHEART Registry, Journal of the American Heart Association (2017). DOI: 10.1161/JAHA.117.007123
- Haruko Nakano,Itsunari Minam,Daniel Braas,et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis, eLife (2017). DOI: 10.7554/eLife.29330