Over the past 3 million years, the evolution of larger brains has played an important role in our ability to think, solve problems, and develop cultures. However, it has always been a mystery for us to become genetic changes in the human brain. In two papers published in Cell, two groups of researchers identified a gene family, NOTCH2NL, which appears to play an important role in the human-specific cortical development and may become a driving force for the evolution of our brain. The NOTCH2NL gene extends the differentiation of cortical stem cells into neurons, resulting in more neurons produced throughout the development process. These genes are only found in humans and are highly expressed in neural stem cells in the human cerebral cortex and are located in a genomic region associated with neurodevelopmental disorders. As the author of the first paper, David Haussler, a bioinformatics scientist at the University of California, Santa Cruz, said, “Our brains have become threefold larger by expanding certain functional areas of the cerebral cortex, and this must be why we become humans. The foundation of the problem is that there are really no more interesting scientific issues than discovering and hacking us into our own mysterious genetic changes.
A team led by Haussler, senior author Frank Jacobs of the University of Amsterdam in the Netherlands, and senior author Sofie Salama of the University of California, Santa Cruz, realized that they were able to detect NOTCH2NL in human cells but could not detect it in macaque cells. They compared genes that are expressed during human and macaque brain development in stem cell-derived models. By studying NOTCH2NL, they also did not observe it in orangutans and found an inactive truncated version of NOTCH2NL in our closest relatives to gorillas and chimpanzees.
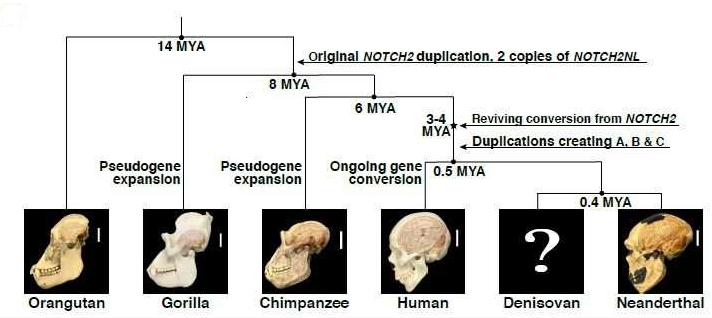
The reconstruction of the NOTCH2NL gene’s evolutionary history reveals that a process called gene conversion may be responsible for repairing the non-functional version of NOTCH2NL, which originally appeared as a partial duplication of an important neurodevelopmental gene (NOTCH2). This kind of repair happens only in humans – they estimate it happened between 3 and 4 million years ago, and about the same time, fossil records suggest that the human brain has begun to expand. After it was repaired, before we were separated evolutionarily from our common ancestor with Neanderthals, NOTCH2NL was duplicated twice.
In the second paper, a team led by Pierre Vanderhaeghen, a developmental biologist at the Free University of Brussels in Belgium, discovered NOTCH2NL from another relevant direction, that specifically looking for human-specific genes that are active during fetal brain development. Vanderhaeghen said, “One of the ultimate goals of researchers like us is to discover what causes larger brains, especially the cerebral cortex, during human development and evolution. It is easy to speculate on the relatively rapid evolution of the human brain. The newly evolved human-specific genes may help shape our brain in a species-specific manner.”
Finding human-specific genes involved in brain development proved to be challenging because these genes are often rarely annotated in the genome database, which makes it difficult to distinguish them from the more common genes found in other species. In order to specifically and highly sensitively detect human-specific genes in the human fetal cerebral cortex, the Vanderhaeghen team developed a custom RNA sequencing assay. This allowed them to identify 35 human-specific genes that are active during the development of the human cerebral cortex, including the NOTCH2NL gene.
The Vanderhaeghen team pays special attention to NOTCH2NL because its ancestral gene NOTCH2 plays an important role in controlling whether cortical stem cells produce neurons or regenerate more stem cells. They found that artificial expression of NOTCH2NL in mouse embryos increased the number of stem cells in the mouse cortex. To better understand the role of these genes in the human body, they used the in vitro model of cortical development generated by human pluripotent stem cells to explore NOTCH2NL function.
In this model, they found that NOTCH2NL can significantly increase the number of cortical stem cells, which in turn produces more neurons. This feature is expected to distinguish between human and non-human cortical neurogenesis. Vanderhaeghen said, “For stem cell, they could either regenerate two stem cells, produce two neurons, or produce one stem cell and one neuron. What NOTCH 2NL does is to make this fate decision slightly biased toward regenerating stem cells. In this way, they will continue to produce more neurons later. This is a small early effect, but produces larger results in the later period, which often occurs during evolution.”
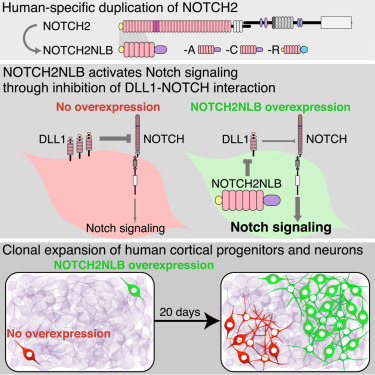
The Haussler team studied what happens when NOTCH2NL doesn’t express. They remove it from human stem cells and use them to develop cortical patches known as organoids. In the organoids derived from NOTCH2NL missing stem cells, they differentiated more rapidly, but the formed organoids were smaller. Jacobs said, “If you miss NOTCH2NL, it will cause the cortical stem cells to differentiate into neurons prematurely, but at the same time these very important stem cell banks will deplete.”
The location of NOTCH2NL on the genome was incorrectly mapped before this, and this time the correct rendering further supports its role in the human brain. It is known that a duplication or deletion of a genomic region known as 1q21.1 leads to macrocephaly or microcephaly, respectively, and is associated with a series of neurodevelopmental disorders, including attention deficit hyperactivity disorder, autism spectrum disorder, and intellectual disability. The Haussler team studied 11 patients who had errors in this area and found that NOTCH2NL indeed replicate or miss in rearrangements associated with larger and smaller brain sizes. Haussler said, “We really hope that this gene is in the pathological region of 1q21.1 because it is logical, but in the wrong reference genome, it is not. Then we found new data, and we realize that there is an error in reference genome! When you hope that something that seems to be false is true, this is very rare, but the results confirm that it is indeed true. I think this kind of thing will not happen again in my career.”
Given that, in a sense, NOTCH2NL is an evolutionary balance between greater brain and susceptibility to 1q21.1 disease, these researchers are quick to point out that there are also many health changes. Salama said, “This may allow us to get a larger brain. This is a gospel. However, this is also a curse, because we may produce these reorganization events that may not be good. But when we sequenced this gene in the individual, we found that it had multiple different alleles. These variations may have resulted in subtle differences and plasticity, what makes human become human.”
When it comes to NOTCH2NL, there are still many unknowns. The Haussler team pointed out that they can only study the genome of a small number of patients, and their organoid model does not address the later stages of cortical development. NOTCH2NL may play a more important role in the later stages of cortical development. Another important question that the Vanderhaeghen team wants to address is what role other human-specific genes (especially those found in the 1q21.1 region or other genomic regions associated with brain diseases) that are found during brain development play. Although both teams were able to verify that NOTCH2NL was involved in the well-studied Notch signaling pathway, Vanderhaeghen acknowledged that the exact mechanism by which NOTCH2NL breaks the balance between differentiation and regeneration remains uncertain.
Vanderhaeghen said, “What’s surprising is that there are many signaling pathways that control embryonic development and are completely conserved between species. The Notch signaling pathway is one of the oldest signaling pathways. You can find it on every animal you studied. It has been used by developing embryos since the animals existed. However, especially in the human pedigree, this signaling pathway produces new functions through NOTCH2NL.”
Jacobs said, “This locus is unstable throughout the evolutionary process, so the repair of these non-functional NOTCH2NL genes may occur at any time. It may have occurred in the primate lineage and had a huge impact on the brain development, but it’s not. It’s linked to luck or chance, which has always attracted me: How do you find something with such an important function in the ambiguous part of our genome and it was used by our species to select these important properties?”
Reference
Ian T. Fiddes15, Gerrald A. Lodewijk15, Meghan Mooring et al. Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell, 31 May 2018, 173(6):1356–1369, doi:10.1016/j.cell.2018.03.051
Ikuo K. Suzuki, David Gacquer, Roxane Van Heurck et al. Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta/Notch Regulation. Cell, 31 May 2018, 173(6):1370–1384, doi:10.1016/j.cell.2018.03.067