Imagine: your heart is failing to work, and a replacement is deadly in need. Consistently, your family waits anxiously for a call from the hospital for the found of donor. At that point, one day, the call comes. In your fervor you scarcely hear what the doctor on the flip side of the line is letting you know. There’s something you ought to know, she says. The contributor is not human. It’s a pig.
Actually, it’s not a fantasy. In 2014, scientists announced that a pig’s heart had survived for more than one year after it was transplanted into a baboon, being a proof for the assumption above. This project, headed by Muhammad Mohuiddin from the US National Heart, Lung and Blood Institute in Bethesda, Maryland, supports the majority’s view that animal organs can provide help for those struggling to wait for organ transplants.
While, such surgery would arise kinds of thorny issues about how we will define “a fully human” in the future? If we can accept pig’s heart, then what other parts could be utilized to strengthen human health? Will the transplant of nonhuman organs change our relationship with animals or even each other?
Nowadays, however, primates are no longer considered to be viable donors, because of issues such as diseases transmission risk and the ethical considerations of primate research. Moreover, human body may probably reject the foreigners. “Immunological rejection, is the major obstruction to xenotransplantation.” said Mohuiddin. Pigs, on the other hand, have been proven to be better donors, at least it works in baboons. A pig’s heart is anatomically similar to a human’s, and it posses less disease risk and the animals grow fast, making them an ideal substitute.
Scientists, for the first time, successfully produced human-pig synthetic organisms. Pig embryos injected into human stem cells cultivated human heart, liver and even nerve cell tissue, being considered a major step forward for xenotransplantation. But the embryos were destroyed four weeks later for moral controversy.
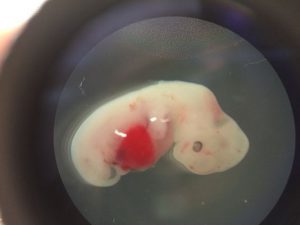
This research was published on Cell few days before. Scientists from the California Solke Institute, firstly used genetic editing techniques to remove deoxyribonucleic acid (DNA) responsible for the growth of specific organs from pig embryos, and then injected into the embryo a human-Induced pluripotent stem cell (also known as “IpS cells”), and then implanted the “Chimera” into the female pig uterus for about 4 weeks. Tests found that the Chimera began to shape, and human stem cells developed into precursor cells of heart, liver, nerve cells and others.
The results of the study were a major step forward in cross-species synthesis studies, but the success rate was still low, for only 186 of the 2,075 embryos implanted in stem cells survived for 28 days. The research team believes that the technology is still in its infancy, and there is still a great distance from the days of cross-species organ transplantation, and the growth process is affected by other factors due to the different evolutionary stages of different species. In other words, the experiment is easy to deviate; to imitate the creation process of is not easy.
According to the British Daily Mail, the United Kingdom will be the first country to use modified pig organs to treat newborn birth defects. Britain will use this method next year to treat 10 patients with oesophagus atresia.
The esophagus and the stomach of Esophageal atresia patients are not connected, and they feel difficult to swallow, resulting in other more serious problems, including asphyxia and pneumonia. In the UK, about 250 children with esophageal atresia are born each year, and 90% are treated in a simple way.
Esophageal atresia can be diagnosed early in the fetus for 20 weeks. Paolo De Coppi, the main developer of the method, says pig organs have been used for heart valve replacement for many years, but the newly developed method is “brand new”.
Pig organs transplanted to the body of are modified by the body’s own stem cells. Different sizes of esophagus used in Surgery are from the British farm. Before transplanting, these organs are cleared, and reprogrammed with the use of the patient’s stem cells, to avoid rejection after transplantation. Stem cells are collected from the muscles of the child and the residual esophagus of the newborn, and the entire tissue work takes about eight weeks. The cost of treatment is expected to be £100,000/time, but the doctor thinks the cost will drop.
Smithfield Foods is the world’s largest pig producer and pork supplier. At present, the company has opened up a new business scope, reaching out to the field of human organ transplantation.
In mid-April this year, Smithfield said the company will seek more possibilities in the medical field, including transplanting pig tissues and organs to the human body. “As a food company, we have the responsibility to ensure that the pig is kept to the greatest extent possible to minimize waste,” says Courtney Stanton, vice president of Smithfield Bioscience. “There are usually many parts of the pig we do not eat, such as the heart. So hope we can “turn waste into treasure”, and put them into medical use for the benefit of more people.”
In April this year, Smithfield, worked with a public-private affiliated engineering firm that received $80 million from the US Department of Defense. In addition, the company has also become an independent department—Smithfield Bioscience, which specializes in medical matters.
The company slaughters about 16 million pigs a year and plans to connect directly with researchers and medical institutions that study pig organ transplants and sell them directly the main organs and pig skin needed for the experiment.
According to the Health Resources and Services Authority (HRSA), there are currently 118,000 people waiting for organ transplants in the United States, with an average of about 22 people die a day in waiting. In 2016, US organ transplant surgery reached 33,500 cases, setting another record.
There are two major barriers to pig organ transplantation: First, the pig’s endogenous retrovirus is embedded in the genome: in the pig body it will not be toxic, but when transplanted into human beings, it will be activated; Second, the Immunological rejection, the recipient’s immune system will attack, destruct and remove the allogeneic tissue or organ once identified.
Smithfield has now begun to sell organs such as porcine pancreas and thyroid, for a variety of medical purposes. At present, the pig raw material market for medical, pet food and non-food use has reached more than $100 billion.
Most of the raw material of pigs sold by the company are used in drugs for the treatment of indigestion and hypothyroidism, etc., but not for the cultivation of human cells.
Smithfield is not the only company involved in pig organ transplantation. Biotech start-up Egenesis also raised $38 million in March of this year. By utilizing genetic editing tools, the company makes pig organs suitable for human transplantation.
Regenerative medicine company Revivicor, also cultivated pigs that have similar gene with humans in a farm in Virginia. The company transferred the human gene into the pig’s liver, kidney and heart.
Stanton said that her team still continues to learn and explore in the use of these new technologies, “At present our experiment is still in its infancy, but in the future we hope we can do help for more organ transplants, providing solution for more damage-restoration problems.”
