ELISA Protocol
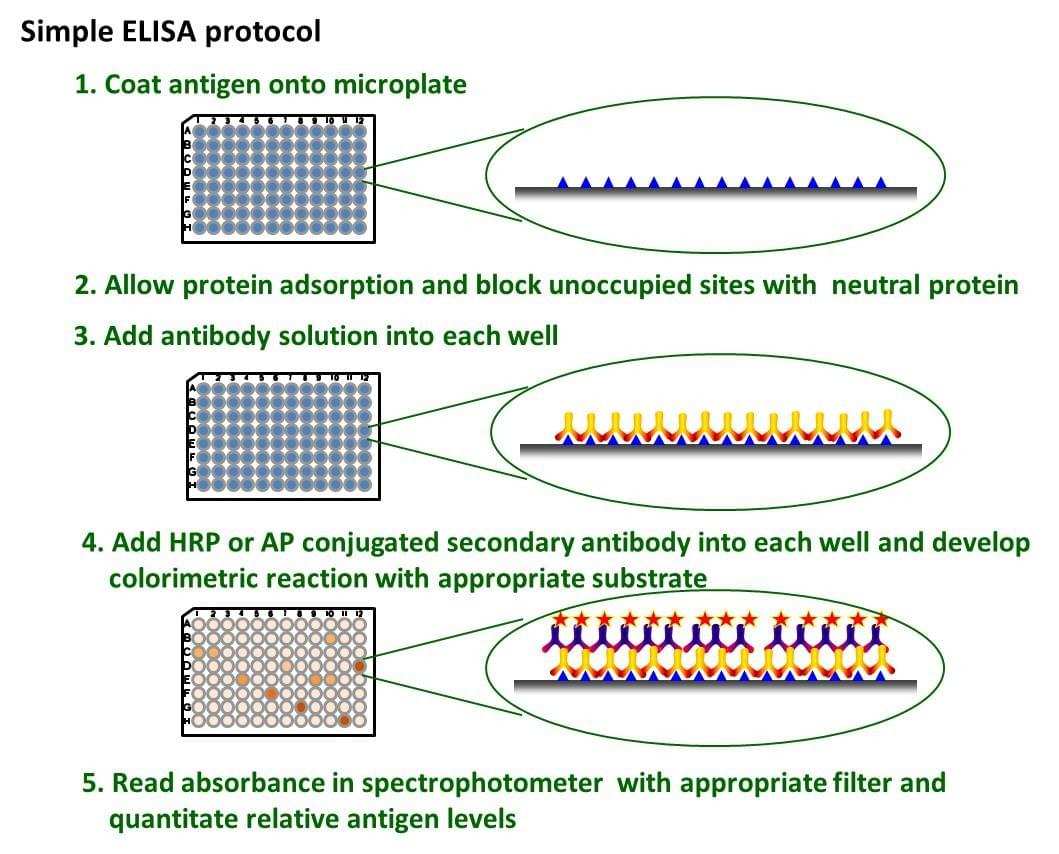
Fig. The schematic diagram of simple ELISA
ELISA procedure
1.Prepare Protein Antigen
- Dilute protein antigen in Bicarbonate ELISA Coating Buffer (BECB).
- Final concentration = [2µg / ml].
- Make 10ml solution for 1 full ELISA plate.
- Protein stocks concentration: = 10µg / µl.
- Use 1µl of protein in 5ml ECB or 2µl in 10ml BECB.
- Use the multichannel pipettor to add coating buffers, protein antigen (Ag), and antibody solutions.
- Coat wells of high binding polystyrene microtiter plates (catalog # S2506710, Immulon Thermoflecton Corporation, Milford, MA, USA) with Ag capture agent (protein) or control blocking buffer (BSA) -50µl of 2µg /ml protein antigen /Coating Buffer solution per well, tap and slowly shake to coat wells.
- Cover with adhesive plastic sheet; incubate at 4°C overnight with slowly shaking.
- The next morning, invert plate over sink to dump supernatant, wrap in stack of paper towels and tap on countertop till no more solution comes off.
- Wash 3 times with 300µl PBST / well (150µl 2x using 96 well washer), wrap in stack of paper towels, tap on countertop until no more water comes off.
2.Blocking Buffer
- Block with 5% BSA in PBST blocking buffer 300µl / well, cover plates with adhesive plastic sheet, incubate for at least 2h at room temperature (RT) with slow shaking.
- Invert plate over sink to dump supernatant.
- Add 1% BSA in PBST: 100µl / well to the four blank wells, the first two wells of rows A and B on each plate.
3. Add Serum (Primary Antibody)
- All serum samples and negative and positive controls are stored in -70 °C.
- Vortex for 30 s to a minute before diluting a sample.
- Prepare fresh serum dilution (negative control, positive control, and samples) (1:1000) in 1% BSA in PBST in 10ml plastic tubes.
- You will need 100µl diluted serum / well, and each is done in duplicate, so:
- 3000µl total at 1:1000 = 3µl serum in 2997µl 1%BSA. Just need 600µl dilution for the negative control, 600µl dilution for the positive control and 200µl dilution samples for test 1% BSA as a blank.
- Vortex for 30 s.
- Load 100µl diluted serum/well, cover plate with adhesive plastic sheet incubating for two hours at RT, while slowly shaking (150-200 rpm).
- Invert plate over sink to dump diluted serum (primary antibodies).
- Wash six times with 300µl PBST using Modern plate washer; each wash has 15 s intervals.
4. Add Secondary Antibody
- Dilute HRP conjugated secondary antibodies: Goat Anti-Human IgG or IgA, 1:10,000 in 1% BSA in PBST.
- You will need 100µl / well, but make a little extra to account for pipetting errors.
- You will have 96 wells total ≈9,600µl, but round up to 10,000µl or 10ml diluted secondary antibodies / plate 1µl in 9.999ml 1:10,000 dilution.
- Add 100µl diluted secondary antibodies / well, cover plates with an adhesive plastic sheet; incubate for one hour at RT with slowly shaking (150-200 rpm).
- Take peroxidase substrate out of refrigerator, and allow it to equilibrate to RT.
- Invert plate over sink to dump secondary antibodies.
- Wash extensively, 6 times using Modern plate washer, with 300µl PBST, as before.
5. Develop Plates Measure Optical Density
- 5.1. After the final washing develop plates with 100µl / well of RT peroxidase substrate, develop for 5 min for IgG and 30 min for IgA; protect from light at this time. Then add 50µl / well H2SO4 stop solution.
6. Measure OD at 450nm in an ELISA plate reader
- 6.1. Use mean values of reactivity of the duplicate samples.


