HSPA1A
-
Official Full Name
heat shock 70kDa protein 1A
-
Overview
HSPA1A, a stress response protein belongs to the HSP70 family. In cooperation with other chaperones, Hsp70s stabilize preexistent proteins against aggregation and mediate the folding of newly translated polypeptides in the cytosol as well as within organelles. These chaperones participate in all these processes through their ability to recognize nonnative conformations of other proteins. They bind extended peptide segments with a net hydrophobic character exposed by polypeptides during translation and membrane translocation, or following stress-induced damage. In case of rotavirus A infection, serves as a post-attachment receptor for the virus to facilitate entry into the cell. -
Synonyms
Heat Shock 70 kD Protein 1A; HSP70-1; HSPA1;
- Recombinant Proteins
- Cell & Tissue Lysates
- Protein Pre-coupled Magnetic Beads
- Cattle
- Human
- Mouse
- Pongo abelii (Sumatran orangutan)
- Rat
- Sumatran orangutan
- E. coli
- E. coli.
- E.coli
- HEK293
- HEK293T
- Insect cell
- Insect Cell
- Insect Cells
- Mammalian cells
- Sf9 Insect Cell
- C
- His
- Flag
- GST
- His (Fc)
- Avi
- Strep
- Myc
- DDK
- Myc|DDK
- N/A
- N
- No tag
- Background
- Quality Guarantee
- Case Study
- Involved Pathway
- Protein Function
- Interacting Protein
- HSPA1A Related Articles
What is HSPA1A protein?
HSPA1A (heat shock protein family A (Hsp70) member 1A) gene is a protein coding gene which situated on the short arm of chromosome 6 at locus 6p21. HSPA1A, a stress response protein belongs to the HSP70 family. In cooperation with other chaperones, Hsp70s stabilize preexistent proteins against aggregation and mediate the folding of newly translated polypeptides in the cytosol as well as within organelles. These chaperones participate in all these processes through their ability to recognize nonnative conformations of other proteins. The HSPA1A protein is consisted of 641 amino acids and its molecular mass is approximately 70.1 kDa.
What is the function of HSPA1A protein?
The function mainly involves the folding, assembly and deaggregation of proteins in the cell, and helps to protect and restore the structure and function of proteins in the cell under stress conditions, so as to maintain the homeostasis of cells under stress. In conclusion, HSPA1A plays an important role in cellular protection, maintaining the structure and function of proteins, helping cells adapt to changes and stress in the external environment, and ensuring the homeostasis balance of cells. HSPA1A can also affect important biological processes such as apoptosis and immune response, and play an important role in cell survival and immune response.
HSPA1A Related Signaling Pathway
HSPA1A can regulate the activity of JAK/STAT signaling pathway and affect the biological processes of cell proliferation, differentiation and apoptosis. HSPA1A is related to Wnt/β-catenin signaling pathway, which can regulate the stability of extracellular protein β-catenin and affect cell proliferation and differentiation. There are also NF-κB, MAPK/ERK, and PI3K/AKT signaling pathways.
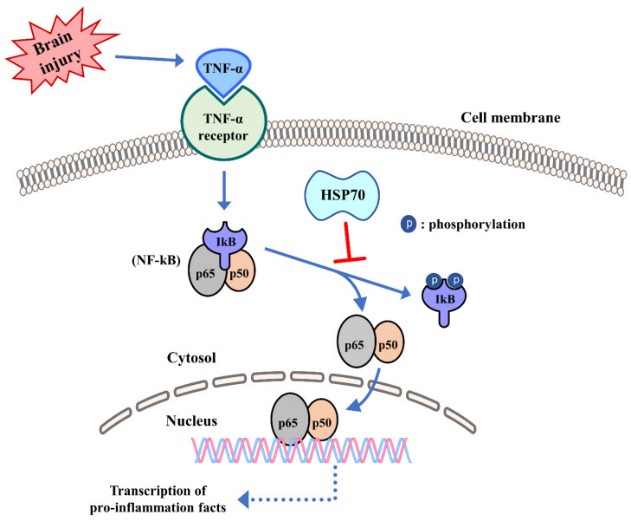
Fig1. Influence of HSPs in innate immunity. Following ischemic stroke, HSPs have been shown to inhibit the activation of the transcription factor NF-kB and to prevent its nuclear translocation. (Jong Youl Kim, 2020)
HSPA1A Related Diseases
HSPA1A Related Diseases
HSPA1A has been found to be abnormally expressed in a variety of cancers, including breast cancer, prostate cancer, and lung cancer. It is involved in the proliferation, invasion, metastasis and drug resistance of cancer cells, and it is also associated with a variety of diseases, including neurological diseases, cardiovascular diseases and inflammatory diseases.
Bioapplications of HSPA1A
Since HSPA1A plays an important role in the occurrence and development of a variety of diseases, such as cancer, diabetes, neurodegenerative diseases, etc., it has potential clinical application prospects, such as as a potential drug target or therapeutic strategy. Now it is more used to study the life activities of cells related scientific research.
High Purity
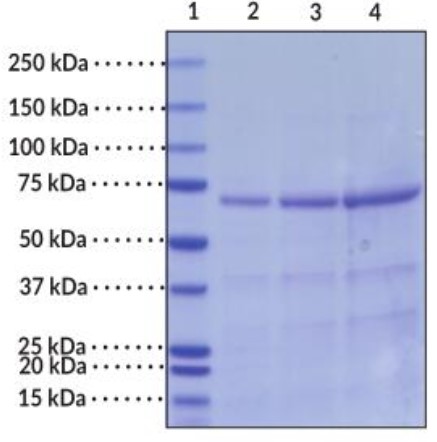
Fig1. SDS-PAGE (HSPA1A-012H) (PROTOCOL for western blot)
.
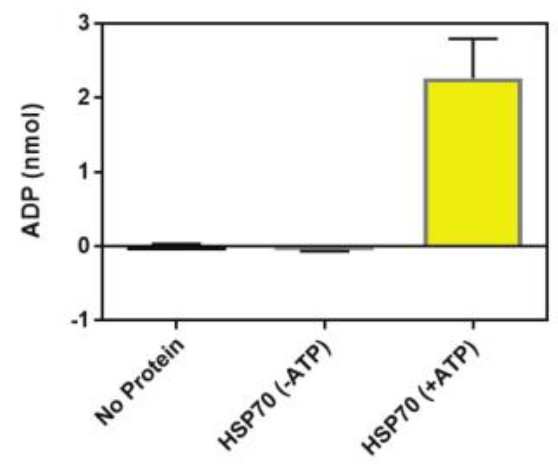
Fig2. Activity Data. (HSPA1A-012H)
Case study 1: Dexun Hao, 2021
Chronic obstructive pulmonary disease (COPD) is characterized by airway obstruction and progressive lung inflammation. As the primary ingredient of a traditional Chinese medical herb, Baicalin has been previously shown to possess anti-inflammatory abilities. Thus, the current study aimed to elucidate the mechanism by which baicalin alleviates COPD.
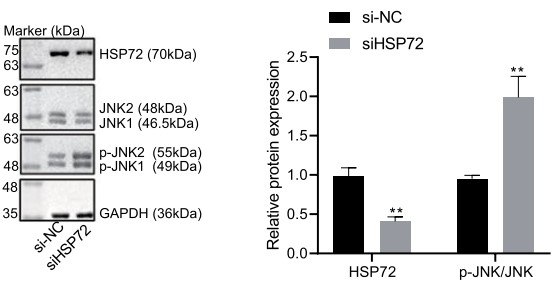
Baicalin was adopted to treat cigarette smoke in extract-exposed MLE-12 cells after which cell viability and apoptosis were determined. A COPD mouse model was constructed via exposure to cigarette smoke and lipopolysaccharide, baicalin treatment. The effect of HSP72 and JNK on COPD following treatment with baicalin was assessed both in vivo and in vitro by conducting loss- and gain- function experiments. Baicalin treatment increased HSP72 expression, while its depletion reversed the effect of baicalin on COPD. HSP72 inhibited the activation of JNK, while JNK activation was found to inhibit the effect of baicalin on COPD.
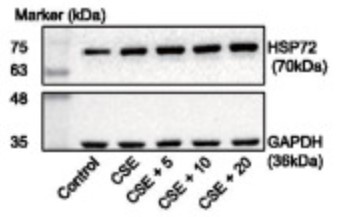
Fig1. The expression of HSP72 normalized to GAPDH was analyzed using immunoblotting after MLE-12 cells were treated with baicalin for 24 h and stimulated by CSE for 2 h.
Case study 2: Chieh-Ting Fang, 2020
The heat shock protein 70 (HSP70) is a conserved molecular chaperone and proteostasis regulator that protects cells from pharmacological stress and promotes drug resistance in cancer cells. In this study, the researchers found that HSP70 may promote resistance to anticancer drugs that target the mitotic kinesin, Eg5, which is essential for assembly and maintenance of the mitotic spindle and cell proliferation. The data show that loss of HSP70 activity enhances Eg5 inhibitor-induced cytotoxicity and spindle abnormalities. Furthermore, HSP70 colocalizes with Eg5 in the mitotic spindle, and inhibition of HSP70 disrupts this colocalization. Using ground state depletion microscopy followed by individual molecule return (GSDIM), they found that HSP70 inhibition reduces the size of Eg5 ensembles and prevents their localization to the inter-polar region of the spindle. In addition, bis(maleimido)hexane-mediated protein-protein crosslinking and proximity ligation assays revealed that HSP70 inhibition deregulates the interaction between Eg5 tetramers and TPX2 at the spindle pole, leading to their accumulation in high-molecular-weight complexes.
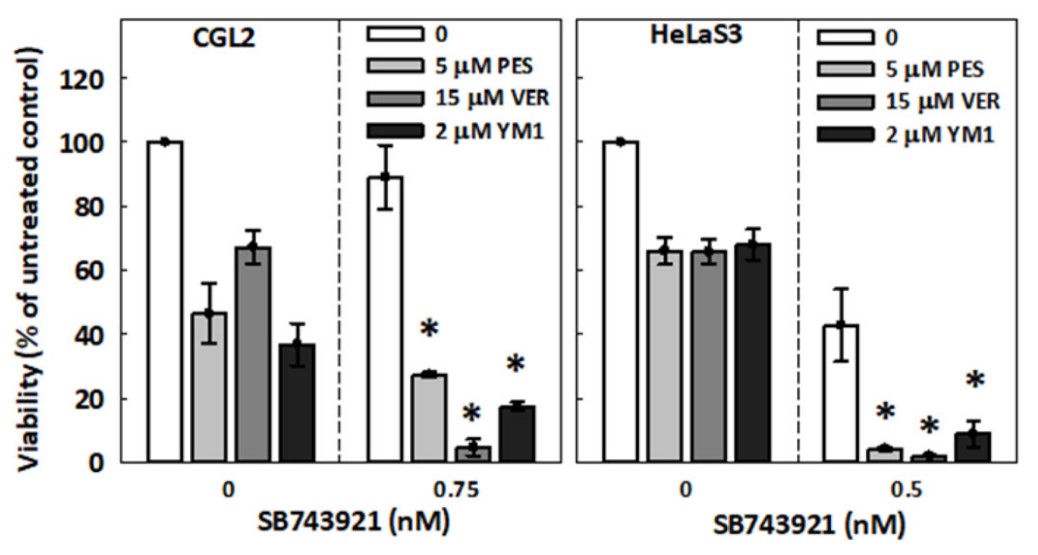
Fig3. HSP70 chaperone activity attenuates cell sensitivity to Eg5 inhibitors. HSP70 inhibitors enhanced the cytotoxicity of SB743921.
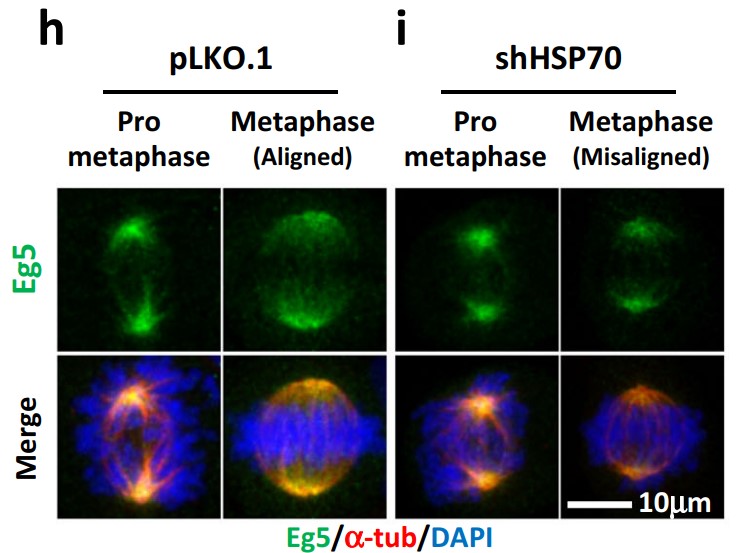
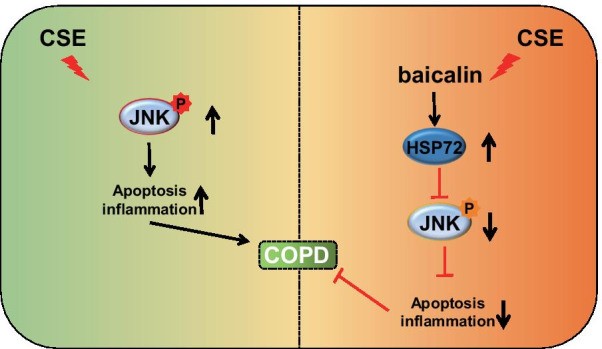
Fig1. Aqueous CSE stimulation promotes the activation of the JNK pathway thereby promoting the development of COPD, while baicalin can up-regulate the expression of HSP72, which in turn inhibits the activity of the JNK pathway and impedes the development of COPD. (Dexun Hao, 2021)
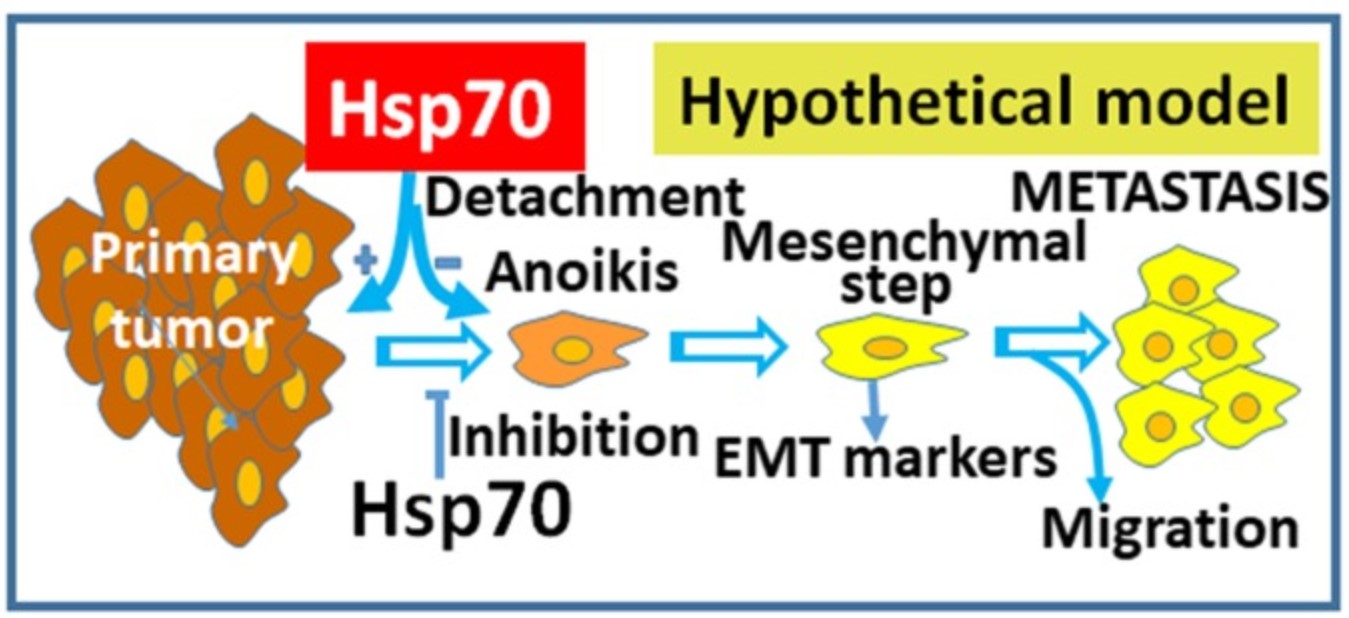
Fig2. Schematic representation of the model of Hsp70 anti-metastatic function on cancer cells. (Panagiota Kasioumi, 2019)
HSPA1A involved in several pathways and played different roles in them. We selected most pathways HSPA1A participated on our site, such as Spliceosome, MAPK signaling pathway, Protein processing in endoplasmic reticulum, which may be useful for your reference. Also, other proteins which involved in the same pathway with HSPA1A were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Spliceosome | SRSF1A;PUF60A;DDX23;SRSF7B;HNRNPM;PRPF3;PPIH;RBM17;PRPF40B |
| MAPK signaling pathway | MAPK14B;RAP1AB;FGF6B;PRKACAB;NTRK1;MAPK7;TNF;CDC42;HSPA8 |
| Protein processing in endoplasmic reticulum | EDEM2;SEC24B;WFS1;BAG1;SEC63;DNAJB11;SELS;RAD23AB;DNAJC5G |
| Endocytosis | ITCH;PDGFRA;ARF5;CCR5;FAM21A;GRK4;GRK1A;CLTCB;CDC42L2 |
| Antigen processing and presentation | CD8A;TAP2;NFYC;HLA-DRB4;KIR2DS2;HLA-DPA1;HLA-F;KIR3DL2;H2-Q10 |
| Estrogen signaling pathway | HSP90B1;PRKACB;CALM2;GNAI1;MAPK3;CALM4;HSPA1A;OPRM1;KCNJ9 |
| Prion diseases | C5;C7;MAP2K1;ELK1;MAPK3;IL1A;LAMC1;CASP12;NOTCH1 |
| Legionellosis | BNIP3;TNF;NAIP6;NAIP7;CASP1;CR1;CD14;TLR2;HSPD1 |
| Toxoplasmosis | TGFB1;HLA-DRA;PIK3CA;HLA-DRB1;LAMB1;HLA-DOB;MAPK12;MAPK1;PIK3R1 |
| Measles | CLEC4M;TLR7;TAB2;BBC3;STAT5B;IKBKE;IL4;CCNE1;IFNA1 |
| Influenza A | DNAJB1;IFNA7;IFIH1;PIK3R3;EP300;PIK3R1;XPO1;HSPA6;CCL5 |
| Epstein-Barr virus infection | SPI1;HLA-A;EIF2AK4;POLR3F;IL10RA;YWHAH;EIF2AK3;POLR2F;POLR2J2 |
HSPA1A has several biochemical functions, for example, ATP binding, ATPase activity, ATPase activity, coupled. Some of the functions are cooperated with other proteins, some of the functions could acted by HSPA1A itself. We selected most functions HSPA1A had, and list some proteins which have the same functions with HSPA1A. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| ATP binding | NEK5;MOV10B.2;ACTG2;MCM5;CCT7;DSTYK;TNIKA;ABCB10;MYH11 |
| ATPase activity | HAND2;ABCD1;DNAH11;KIF13B;KIF2A;DNA2;ATAD1;ABCF2;PSMC4 |
| ATPase activity, coupled | HSPA8;HSPA1B;NSF;HSP104;PEX1;VPS4B;HSPA1A;ABCB1A;CCT8 |
| C3HC4-type RING finger domain binding | HSPA1B;DNAJA1;HSPA1A;PINK1;KCNH2;HSPA8 |
| G-protein coupled receptor binding | PROK1;NPB;GNA13B;UCN3L;RSPO2;NMS;RSPO1;GNA12A;INSL3 |
| enzyme binding | RAD9;RARA;TSPAN15;NFKBIA;JUND;TBP;N4BP2L2;HMGA1-RS1;RPA2 |
| heat shock protein binding | DMPK;HSPA6;BAK1;DNAJA3B;PPP5C;HSPA1B;NFKB1;TPR;FKBP4 |
| protein binding | NTM;TCEA1;ATF3;COQ7;RTN4RL2B;DDI1;TBATA;C14orf104;KBTBD7 |
| protein binding involved in protein folding | CLGN;HSPA1B;HSPA1A;PFDN2;CD74;DNAJB8;CALR;CALR3;PFDN1 |
| receptor binding | KNG1;CAV1;WIPI1;NTF3;HCK;MIF;RCHY1;HAO1;GAS6 |
| ubiquitin protein ligase binding | SQSTM1;CUL1B;TPI1;UBE2U;LTBR;BID;RNF20;DAXX;IKBKG |
| unfolded protein binding | TRAP1;HSPA1B;TMEM67;CCT5;ERO1LB;CCT6A;CCT2;SYVN1;NPM1 |
| virus receptor activity | SCARB1;SCARB2;TYRO3;TNFRSF4;AXL;SERPINB4;GYPA;TNFRSF14;MOG |
HSPA1A has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with HSPA1A here. Most of them are supplied by our site. Hope this information will be useful for your research of HSPA1A.
EGFR; STUB1; SNCA; HSP90AA1
- Q&As
- Reviews
Q&As (6)
Ask a questionHSPA1A has multiple associations with other proteins and diseases. For example, it may interact with the p53 protein to affect the occurrence and progression of tumors, and may also be associated with neurodegenerative diseases and be involved in the pathogenesis of diseases such as Alzheimer's disease.
HSPA1A can protect cells from damage through synergistic effects with other molecular chaperones such as HSP70 and HSP40 under stressful conditions. In addition, it can also be involved in the regulation of apoptosis.
Yes, HSPA1A has therapeutic potential. In tumor therapy, drug suppression or gene therapy against HSPA1A may become a new way to treat tumors. At the same time, inhibitors against HSPA1A are also being developed.
Aberrant expression of HSPA1A may be associated with a variety of diseases, especially cancer, neurodegenerative diseases, etc. For example, in tumors such as lung, breast, and colon cancer, HSPA1A expression levels may be abnormally elevated.
Levels of HSPA1A can be detected by methods such as immunohistochemistry, western blotting, and real-time PCR, which can assess the amount of HSPA1A in tissues and cells.
This protein can bind to unfolded proteins to form multimeric complexes that facilitate proper folding and transport of proteins. In addition, it can also work synergistically with other molecular chaperones such as HSP70 and HSP40 to participate in the correct folding and transport of proteins.
Customer Reviews (3)
Write a reviewUsing HSPA1A experiments can obtain consistent results and reduce the uncertainty of experiments.
HSPA1A has good stability and is suitable for long-term storage and use.
HSPA1A can effectively simulate the function of the target protein in vitro.
Ask a Question for All HSPA1A Products
Required fields are marked with *
My Review for All HSPA1A Products
Required fields are marked with *


