A small study of 8 patients in Greece found that the clinically approved anti-inflammatory drug anakinra (anakinra, which is essentially an IL-1 inhibitor) for the treatment of rheumatoid arthritis It can improve the respiratory function of severe COVID-19 (coronavirus disease 2019, 2019 coronavirus disease) patients. The eight patients also suffered from a disease called secondary hemophagocytic lymphohistiocytosis (sHLH), which was characterized by excessive immune system activation and organ failure. One patient did not need mechanical ventilation, after starting treatment with this drug, his condition improved quickly and he was discharged 9 days later. However, the drug did not prevent the death of 3 of the 7 patients who used the ventilator, and it is unclear whether it will increase mortality. The related research results were published online in the journal Cell Host & Microbe. The paper titled “Favorable anakinra responses in severe COVID-19 patients with secondary hemophagocytic lymphohistiocytosis”.
The Corresponding author of the paper, Evangelos J. Giamarellos-Bourboulis, professor of internal medicine at the University of Athens, Greece, said, “These data suggest that anakinra may be a viable treatment in the case of severe COVID-19 with sHLH, thus I support to conduct larger clinical studies to verify this.”
The mortality rate of severe COVID-19 patients hospitalized in the Intensive Care Unit (ICU) is estimated to be between 50% and 65%. The serious complications of COVID-19 are thought to be driven by an inflammatory response, especially through signaling molecules called interleukin 1β (IL-1β) and interleukin 6 (IL-6). The excessive secretion of IL-1β by immune cells called macrophages can lead to sHLH, also known as macrophage activation syndrome, which is characterized by low blood cell counts, excessive coagulation of blood cells, kidney damage and liver dysfunction. Anakinra can inhibit IL-1β signal transduction and has been shown to reduce the mortality of patients with sHLH syndrome by 30%.
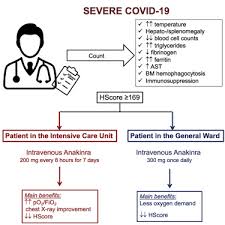
In this new study, these researchers tested whether anakinra can effectively treat patients with severe COVID-19 pneumonia and sHLH. Seven of the eight patients are male, have respiratory failure, use a ventilator in the Greek ICU, and have serious underlying diseases such as heart disease and hypertension. These patients received 200 mg of anakinra intravenously every 8 hours for 7 days. These patients also received anti-malarial drugs hydroxychloroquine and broad-spectrum antibiotics. These researchers monitored their treatment results for 4 weeks.
Anakinra treatment improved most laboratory findings in ICU patients and reduced the signs of sHLH. All patients showed an improvement in respiratory function, as the ratio of arterial blood oxygen partial pressure to inhaled oxygen fraction (PaO2 / FiO2) increased by 15% to 117%. In addition, 6 patients need to use lower doses of blood pressure-increasing drugs. Although three of the seven ICU patients died, previous studies have shown that sHLH causes a mortality rate of up to 67%.
Of these 8 patients, 1 was a non-ICU patient. She was a 71-year-old female patient who was hospitalized with COVID-19 2 weeks after the third chemotherapy cycle. This patient is also taking hydroxychloroquine to treat rheumatoid arthritis. She received anakinra treatment with 300 mg intravenously once a day for 4 days, then 100 mg once a day for 5 days. During the first day she received anakinra treatment, her condition improved, her oxygen demand decreased, her sHLH signs also declined, and she was discharged 9 days after starting treatment. According to these authors, these results suggest that anakinra may prevent the progression of respiratory failure and reduce the need for mechanical ventilation in COVID-19 patients with sHLH.
The first author of the paper, George Dimopoulos of the University of Athens, said, “We believe that anakinra may improve the prognosis of patients with severe COVID-19. Larger clinical trials are still needed to verify these results and confirm the usefulness of anti-IL-1 therapy for COVID-19 at the presence of sHLH.”
Reference
- George Dimopoulos et al. Favorable Anakinra Responses In Severe Covid-19 Patients With Secondary Hemophagocytic Lymphohistiocytosis. Cell Host & Microbe, 2020, doi:10.1016/j.chom.2020.05.007.
- Arthritis drug may improve respiratory function in some patients with severe COVID-19 https://medicalxpress.com/news/2020-05-arthritis-drug-respiratory-function-patients.html