This study reveals how sleep deprivation affects microglia, and finds that TREM2 mediates sleep deprivation-induced microglia overactivation, and β-Amyloid protein (Aβ) deposition increases. Long-term chronic sleep deprivation regulates inflammation and the metabolic reaction of microglia and Aβ in the brain.
With the accelerating pace of society and increasing work pressure, it is sometimes particularly luxurious for modern people to have a good sleep. However, a large number of studies have shown that the quality of sleep is closely related to people’s physical and mental health.
Taking Alzheimer’s disease (AD) as an example, as one of the most common neurodegenerative diseases, its pathological features are β- Amyloid protein (Aβ) deposition and Tau protein aggregation have brought heavy economic and spiritual burdens to countless families and individuals.
It is alarming that animal studies have found that both acute and chronic sleep deprivation can cause increased Aβ deposition in the brain of mice, indicating that sleep deprivation may affect Aβ pathology, in turn, increases the risk of AD. At the same time, insufficient sleep is associated with decreased cognitive ability in the elderly population and is also a risk factor for AD.
David Holtzman’s team from the University of Washington, School of Medicine published a report in the journal Science Translational Medicine titled ‘Sleep deprivation exacerbates microglial reactivity and Aβ deposition in a TREM2-dependent manner in mice’.
This study found that sleep deprivation will prevent microglia from clearing amyloid deposits correctly, thus affecting the progression of Alzheimer’s disease (AD), which helps to explain the long-term observed link between sleep deprivation and neurodegeneration.
The research team suggests that understanding how sleep regulates these complex glial cell interactions will open up new avenues and help determine which molecular pathways may be most suitable for early treatment interventions for sleep disorders and neurodegenerative diseases. The quality of sleep plays a crucial role in human health, and sleep regulation may be a new intervention in the clinical treatment of Alzheimer’s disease.
More and more evidence shows that glia, especially astrocytes, are the key components of the sleep/wake cycle. As the main immune surveillance cells in the central nervous system, microglia can participate in the pathological process of AD by regulating the expression of cytokines, complement proteins, interleukins, and chemokines. Long-term uncontrolled microglia reactions will lead to chronic neuroinflammation, and promote neuronal dysfunction and neurodegeneration.
Recent studies have found that mutations in several immune regulator genes expressed in microglia, including the triggering receptor (TREM2) expressed on myeloid cell type 2, can increase the risk of AD. For example, AD-related R47H function deletion mutation impairs microglia chemotaxis and plaque surrounding aggregation, indicating that the loss of TREM2 function impairs the immune monitoring ability of microglia to recognize and eliminate pathogenic threats.
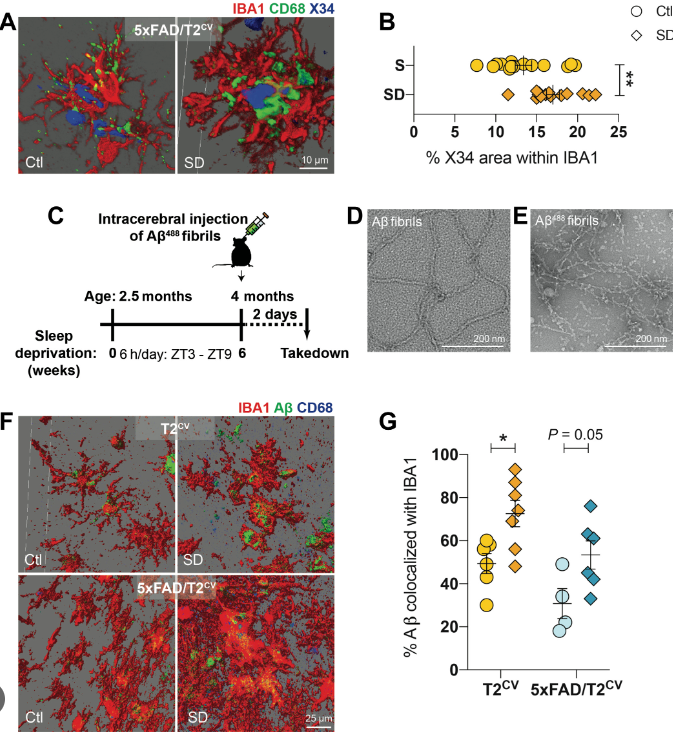
In order to analyze the mechanism of TREM2 in sleep deprivation and AD pathology, the researchers constructed 5xFAD mouse models that express humanized common variants of TREM2 (5xFAD/T2CV), R47H variants with TREM2 dysfunction (5xFAD/T2R47H), and TREM2 knockout (5xFAD/T2KO), respectively, and conducted chronic sleep deprivation experiments (sleep deprivation) on these three kinds of mice. The experimental mice were placed in the automatic sleep debris room. The device included a sliding bar, which was set to move once every 30 seconds for 6 hours every day. The sleep deprivation experiment lasted for 6 weeks.
The results showed that only in the brain of 5xFAD/T2CV mice with sleep deprivation, the deposition of immune positive Aβ is significantly increased, indicating that Aβ deposition induced by sleep deprivation depends on TREM2.
Subsequently, immunofluorescence experiments revealed the presence or absence of Aβ deposition, chronic sleep deprivation can lead to increased responsiveness of microglia in 5xFAD/T2CV mice. Further three-dimensional reconstruction found that after intracerebral injection of Aβ protein, chronic sleep deprivation increased Aβ deposition in microglia of 5xFAD/T2CV mice.
That is to say, sleep deprivation leads to the dysfunctional state of microglia, which makes Aβ increase in microglia. Although the body recruited a large number of reactive microglia, the efficiency of degraded or cleared Aβ protein plaques in the brain has decreased.
It is well known that lysosomes are organelles that help to decompose cell waste. Through transmission electron microscopy, researchers found that chronic sleep deprivation (lack of sleep) led to morphological changes of lysosomes with deformation and swelling of microglia in 5xFAD/T2CV mice. Immunofluorescence and protein immunoblotting experiments found that in the presence of Aβ deposition, chronic sleep deprivation induces significant lysosomal dysfunction, which leads to the damage of Aβ clearance process mediated by microglia, further exacerbating the pathological development of AD.
Transcriptome and proteomics analysis showed that chronic sleep deprivation could cause the specific metabolic transcript profile changes of TREM2 expression, and Aβ deposition is significantly correlated.
In conclusion, this study reveals how sleep deprivation affects microglia, and finds that TREM2 mediates the excessive activation of microglia induced by sleep deprivation, and β- Amyloid protein (Aβ) deposition increases. Long-term chronic sleep deprivation regulates inflammation and the metabolic reaction of microglia and Aβ deposition in the brain plays a crucial role and requires people’s attention.
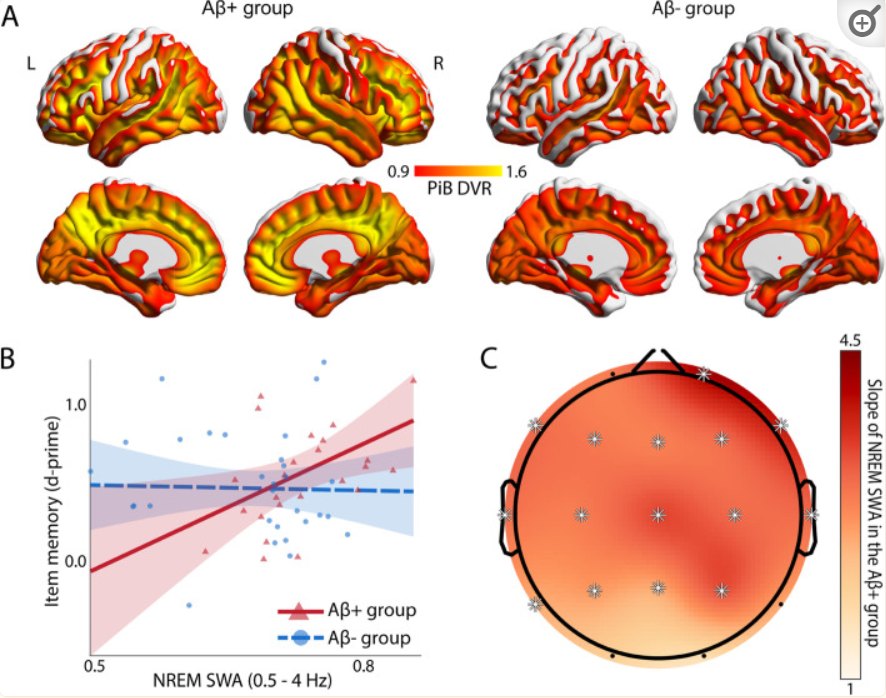
It is worth mentioning that a new study by the University of California, Berkeley, shows that deep slow-wave sleep can be used as a protective factor to prevent the memory ability of Alzheimer’s patients from declining, which brings potential new methods to help alleviate some of the most devastating consequences faced by dementia patients.
This study is titled “NREM sleep as a novel protective cognitive reserve factor in the face of Alzheimer’s disease pathway” and was published in the BMC Biology journal on May 3, 2023. This latest study suggests that deep sleep may help alleviate memory loss in elderly people facing the increased burden of Alzheimer’s disease.
Related Research Area
Neurodegeneration and Neurodegenerative Disease Proteins
Reference
Sleep deprivation exacerbates microglial reactivity and Aβ deposition in a TREM2-dependent manner in mice. April 2023Science Translational Medicine 15(693):eade6285. DOI:10.1126/scitranslmed.ade6285
Zavecz Z, Shah VD, Murillo OG, Vallat R, Mander BA, Winer JR, Jagust WJ, Walker MP. NREM sleep as a novel protective cognitive reserve factor in the face of Alzheimer’s disease pathology. BMC Med. 2023 May 3;21(1):156. doi: 10.1186/s12916-023-02811-z. PMID: 37138290; PMCID: PMC10155344.