Recently, researchers from the University of Copenhagen in Denmark have discovered how certain types of proteins keep damaged DNA stable, thereby preserving its function and integrity. The new findings also explain why people with congenital or acquired defects in certain proteins cannot keep their DNA stable and develop diseases such as cancer. The results were recently published in Nature under the title “Stabilization of Chromatin Topology safeguards genome integrity”.
Every day, cells in the body divide millions of times, and to maintain their identity, it requires the mother cell to transmit the complete genetic information to the daughter cell without error. This is not a small task, because our DNA is constantly attacked by the environment and the cell’s own metabolic activities. The result is that DNA strands can break at least once in each cell division cycle, and this frequency can be increased by certain lifestyles (e.g., smoking) or congenital DNA repair defects. This leads to irreversible genetic damage and ultimately to diseases such as cancer, immunodeficiency, dementia, or developmental defects.
Today, these researchers have discovered how certain proteins coordinate the repair of damaged DNA, thereby ensuring that it remains stable across generations and preventing collateral damage to adjacent undamaged DNA.
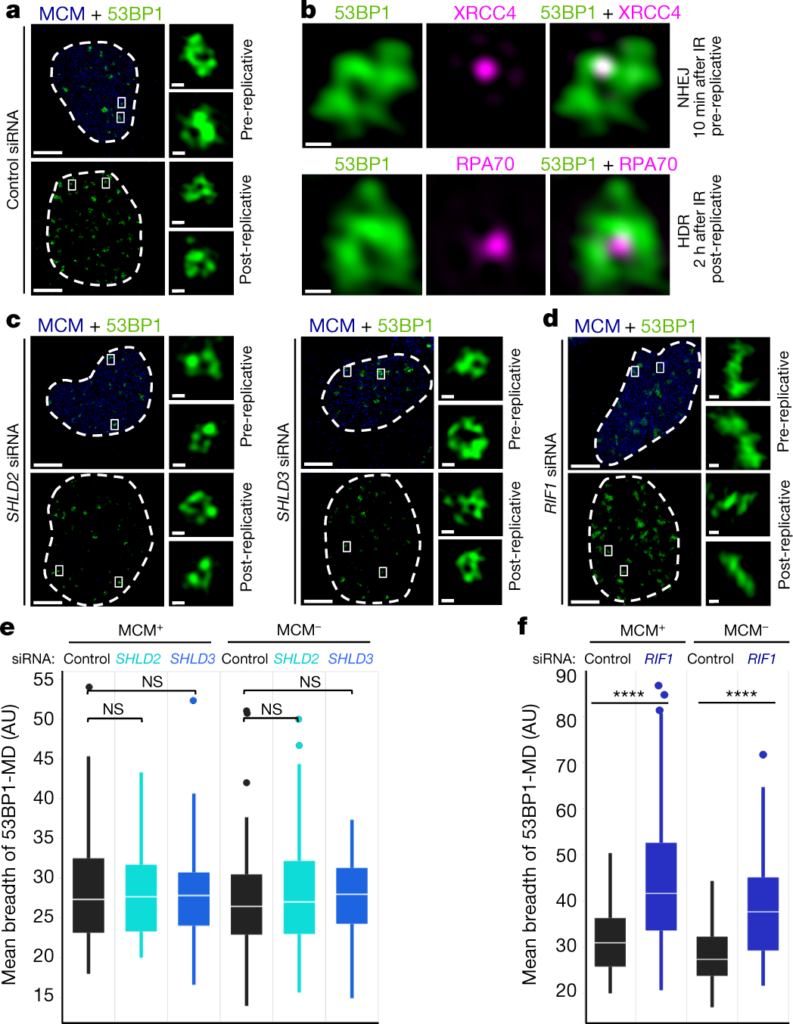
In brief, two proteins called 53BP1 and Rif1 are involved in building a three-dimensional “scaffold” around the broken DNA strand. This scaffold subsequently enriches locally for specialized repair proteins that are in short supply and are required for error-free repair of DNA.
Professor Jiri Lukas, the co-corresponding author of the paper and director of the Novo Nordisk Foundation Centre for Protein Research at the University of Copenhagen, said: “This is a unique finding. Understanding the body’s natural defense mechanisms allows us to better understand how certain proteins communicate and repair damaged DNA. This provides an opportunity to better determine how DNA damage causes disease and design drugs to improve treatment for patients whose DNA does not remain stable.”
Prevent Degradation
This study used highly advanced super-resolution microscopy, a technique that allows one to focus on living cells, visualize objects that are approximately one-thousandth the width of the hair, and track how this protective protein scaffold assembles and grows around broken DNA.
“It’s like sticking a patch on a fractured leg; it keeps the fractured part stable and stops the damage from getting worse and not healing,” said Fena Ochs, first author of the paper and a postdoctoral researcher at the Novo Nordisk Foundation Center for Protein Research at the University of Copenhagen.
Recruitment of Repair Proteins
So why is this discovery so novel? The previous hypothesis was that proteins such as 53BP1 and Rif1 act only in the closest neighborhood of DNA breaks. However, with the help of super-resolution microscopy, these researchers were able to observe that error-free repair of broken DNA requires larger structures.
“Roughly speaking, the difference between the proportion of protein scaffolds and DNA break sites is equivalent to a basketball and a pin,” says Fena Ochs.
According to these researchers, the fact that this supportive protein scaffold is much larger than the DNA break site highlights the importance of the cell to stabilize not only the DNA damage but also the surrounding environment. This preserves the stability of damaged sites and their nearby regions and increases the chances of attracting highly specialized repair proteins in the cell for accurate repair.
These proteins from the so-called Shieldin network were also recently identified by researchers at the Novo Nordisk Foundation Center for Protein Research.
Lack of stent may lead to diseases such as cancer
One of the most significant benefits of basic research, such as this new study, is that it provides scientists with molecular tools to simulate and thus better understand what happens during the production of a disease.
When these researchers prevented cells from establishing this protein scaffold around the broken DNA, they observed rapid disassembly of most of the adjacent chromosomes. This causes DNA-damaged cells to start trying to repair themselves, but this strategy is often futile and exacerbates the destruction of genetic material. This may explain why people lacking these scaffold proteins are prone to diseases caused by DNA instability.
Reference:
1.Fena Ochs et al. Stabilization of chromatin topology safeguards genome integrity. Nature, 2019, doi:10.1038/s41586-019-1659-4.
2.Researchers find ‘protein-scaffolding’ for repairing DNA damage, https://phys.org/news/2019-10-protein-scaffolding-dna.html