Cell Cycle Checkpoint
Life is a continuous process that is passed from one generation to the next. The life of a cell begins with the division of a parent cell, ending with the formation of its daughter cells, or the death of the cell. The daughter cell formation is usually used as a marker for the end of a cell division. The cell cycle refers to the entire process that the cell undergoes from the completion of one division to the end of the next division. In this process, the cell's genetic material replicates and is equally distributed to both daughter cells. It is divided into two phases: interphase and mitotic (M) phase.
Phases
The interphase is divided into three phases, namely the early stage of DNA synthesis (G1 phase), the DNA synthesis phase (S phase), and the late DNA synthesis phase (G2 phase). The mitosis of cells needs to undergo prophase, metaphase, anaphase, and telophase, which is a continuous process from one parent cell to two daughter cells.
| State | Phase | Description |
| Resting | G0 phase (Gap 0) | A phase where the cell has left the cycle and has stopped dividing. |
|
Interphase |
G1 phase (Gap 1) | A period from mitosis to DNA replication, also known as the pre-synthetic period. RNA and ribosomes are synthesized mainly in this period. It is characterized by active metabolism, rapid synthesis of RNA and protein, and a significant increase of cell volume. The main significance of this period is to prepare the material and energy for the next phase of DNA replication. |
| S phase (Synthesis) | It is the DNA synthesis phase. In this phase, in addition to the synthesis of DNA, histones must also be synthesized. Enzymes needed for DNA replication are synthesized during this period. | |
| G2 phase (Gap 2) | It is the late phase of DNA synthesis and is the preparation phase for mitosis. During this period, DNA synthesis was terminated and a large number of RNA and proteins were synthesized, including tubulin and maturation factors. | |
|
M phase (Mitosis) |
Prophase | Chromosomal filaments are highly spiraled and gradually form chromosomes. The chromosomes are short, thick and strongly basophilic. The two centrosomes move in opposite directions, forming two poles in the cell. Then they begin to synthesize microtubules with the centriole as the starting point, forming a spindle. As the nucleolus grows helix, the nucleolus gradually disappears. The nuclear envelope begins to disintegrate into a discrete vesicular endoplasmic reticulum. |
| Metaphase | Cells become spherical, the nucleolus and nuclear envelope have completely disappeared. The chromosomes are all moved to the equatorial plane of the cell. Microtubules from the poles of the spindle attach to the centromere of each chromosome. | |
| Anaphase | Due to the activity of the spindle microtubules, the centromere is split longitudinally, the two chromatids of each chromosome are separated and moved in opposite directions, close to the respective centrosomes, and the chromatids are divided into two groups. At the same time, the cells are elongated. And due to the activity of the microfilaments around the cell membrane, the equatorial zone is narrowed and the cells are dumb bell-shaped. | |
| Telophase | Chromatid gradually helixes, chromatin and nucleoli are reappeared, endoplasmic reticulum vesicle combined to form nuclear envelope, the cell equatorial zone is narrowing, and finally completely split into two daughter cells. |
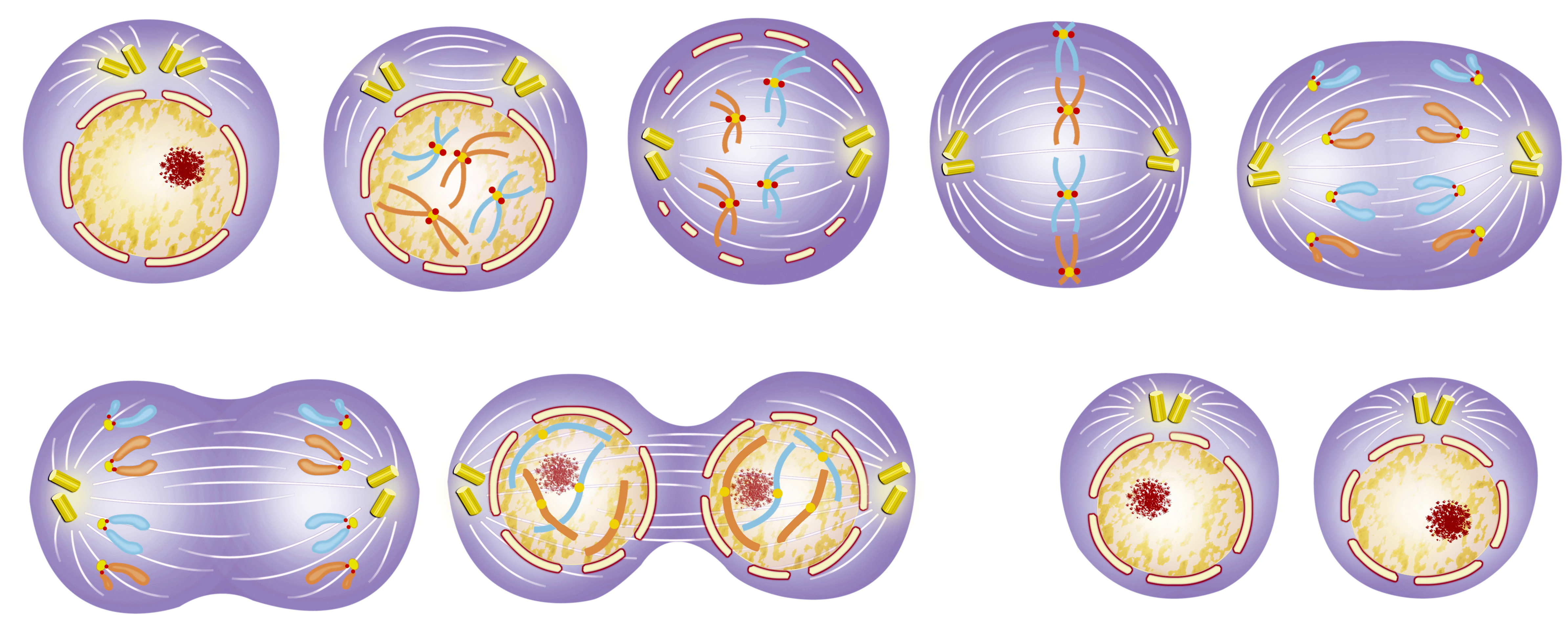
In a proliferating cell population, not all cells are synchronously proliferating. They may have four fates during cell cycle:
- The cells begin the second cycle through again after M phase.
- The cells stop in the G2 phase and are called G2 phase cells (R2), which can be stimulated to enter the cell cycle.
- The cells stop in the G1 phase and are called resting cells or G0 cells. After being stimulated, these cells can still enter the cell cycle and continue to undergo mitosis.
- Cells that no longer divide leave the cell cycle and eventually die.
Cell Cycle and Cancer
Cancer was thought to arise when cell growth exceeds the rate at which cells die, so that cells are dividing at an uncontrollable rate. It is now accepted that cancer is more accurately described as being the product of malfunctions within the regulation of the cell cycle, such that injured or mutated cells which are normally killed, are allowed to progress through the cell cycle, accumulating mutations. Mutations mostly occur in proto-oncogenes and tumor suppressor genes. Proto-oncogenes normally act at different levels of cell proliferation, but can promote tumor growth when mutated. Similarly, mutation of tumor suppressor genes would hinder the inhibition of cell cycle progression, thus facilitating abnormal growth.
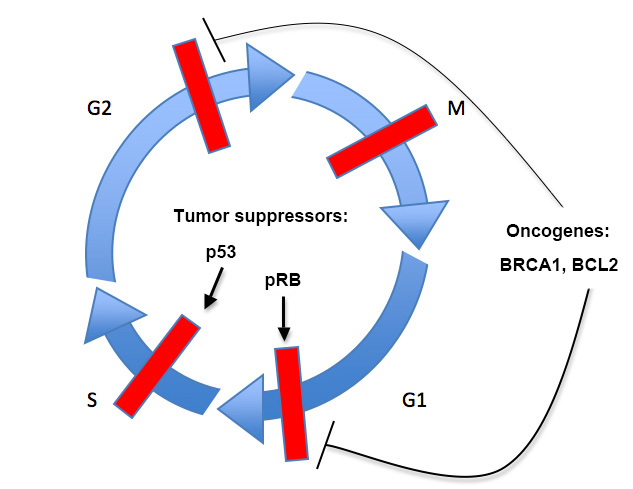
Tumor suppressors, such as p53 and the retinoblastoma protein (RB), function to repress or halt the cell cycle, promote apoptosis, or both. Oncogenes, such as BRCA1 and BCL2, serve to promote cell proliferation or block apoptosis, and are often transcription factors, growth factors or growth factor receptors, or apoptotic regulators. The tumor suppressor p53 is found mutated in almost 50% of all tumors, and the p53 pathway (including upstream regulators or downstream targets) has been proposed to be altered in perhaps all tumors. Another example is the BCL2 oncogene, which is involved in the initiation of almost all follicular tumors and is involved in chronic lymphocytic leukemia and lung cancer. Thus, understanding the cell cycle and its key regulatory components is important to understanding cancer, and perhaps in identifying therapeutic targets.
Under normal conditions, growth-regulating mechanisms endeavor to maintain homeostasis. Homeostasis within a cell, is regulated by the balance between proliferation, growth arrest and apoptosis. Disturbance in the balance between cell growth and death, may result in hyperplasia or neoplasia. Once the stimulus is removed, however, the process of hyperplasia is reversible, whereas in cancer cells, it is irreversible. Cancer cells are characteristically independent of growth stimulus due to mutation of intracellular signal pathways. Such independence facilitates re-entry into the cell cycle, irrespective of positive or negative external stimulus. Fundamentally, all cancers permit the existence of too many cells. However, this cell number excess is linked in a vicious cycle with a reduction in sensitivity to signals that normally tell a cell to adhere, differentiate, or die. This combination of altered properties increases the difficulty of deciphering which changes are primarily responsible for causing cancer.
Reference:
Foster I. Cancer: A cell cycle defect. Radiography, 2008, 14(2):144-149.


