E1 Ubiquitin/Ubl Activating Enzymes
About E1 Ubiquitin/Ubl Activating Enzymes
E1 ubiquitin/Ubl activating enzymes are a group of enzymes involved in the covalent attachment of ubiquitin or ubiquitin-like (Ubl) proteins to target substrates. Ubiquitin and Ubl proteins play critical roles in regulating cellular processes, including protein degradation, DNA repair, signal transduction, and cell cycle progression. Activation of ubiquitin/Ubl proteins by E1 enzymes is the first step in the conjugation pathway and sets the stage for subsequent ubiquitination or Ubl modification events.
Ubiquitin (Ub) and ubiquitin-like (Ubl)-dependent metabolism is a multienzyme process involving the sequential activities of different conjugating enzymes. E1 activating enzymes are central to Ub/UBL conjugation in vivo and in vitro because they initiate the conjugation cascade by activating their respective modifiers. These enzymes are part of the larger E1-like enzyme superfamily that plays a role in the biosynthesis of cysteine, thiamine, molybdenum cofactor (MoCo), and several secondary metabolites. The basic catalytic mechanism of these enzymes involves adenylation and sulfotransfer as well as specific domains and structures that determine the specificity of substrate binding and other related protein-protein interactions. All E1 enzymes possess an adenylation domain (two ThiF homology motifs) that binds and adenylate Ub/Ubl, a catalytic cysteine domain to which Ub/UBL transiently attaches, and a C-terminal Ub fold domain (UFD) recruits E2 enzymes.
Mechanism of E1 Ubiquitin/Ubl Activating Enzymes
Ubiquitin-activating enzyme E1 is responsible for the first step in ubiquitin-protein isopeptide bond formation and is a critical component for the initiation of in vitro conjugation reactions. E1 activates ubiquitin by first adenylating with ATP the C-terminal glycine carboxyl group of ubiquitin and, thereafter, linking this residue to the sulphydryl side chain moiety of a cysteine residue in E1 by forming a high energy thiol ester bond and liberating free AMP. There are two active sites within the E1 molecule allowing it to accommodate two ubiquitin molecules at one time, with a new ubiquitin forming an adenylate intermediate as the previous one is transferred to the thiol site. The activated ubiquitin is then transferred to the lysine of target proteins via the E2/E3 conjugation cascade.
Available Resources of E1 Ubiquitin/Ubl Activating Enzymes
Understanding the precise mechanism of E1 activation is critical to deciphering the regulation and specificity of the ubiquitin/Ubl conjugation system. Further studies on the structure, function, application, and mechanism of E1 enzymes are expected to improve our understanding of protein homeostasis and cellular processes regulated by ubiquitin/Ubl modifications. Creative BioMart offers a range of products and services to support research on E1 enzymes, allowing scientists to explore the complexity of the ubiquitin/Ubl system and its impact on cellular function. We offer a comprehensive range of products including recombinant proteins, cell & tissue lysates, protein pre-coupled magnetic beads, and other products to facilitate E1 ubiquitin/Ubl activating enzymes-related research. The relevant E1 ubiquitin/Ubl activating enzymes are:
In addition to products and customization services, Creative BioMart also provides E1 ubiquitin/Ubl activating enzyme-related signaling pathways, protein functions, interacting proteins, research areas, and other resources to support research on various biological processes and pathways. Please visit the details page for specific available resources for each product. For more information, please contact our team.
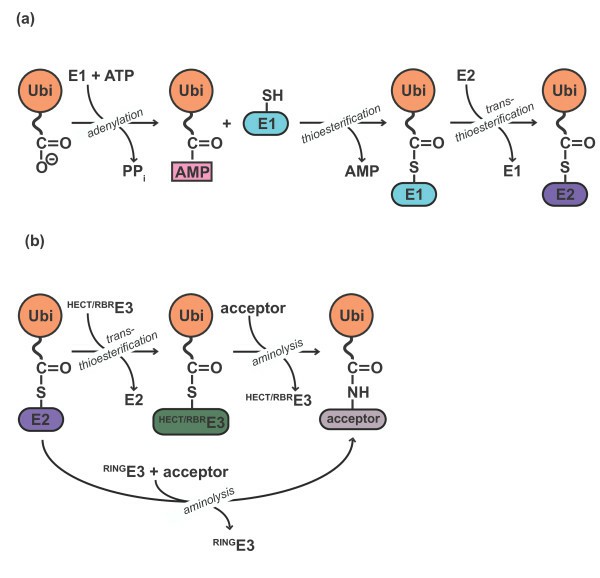 Fig. 1. Ubiquitylation is a multistep reaction. (Lorenz S, et al., 2013)
Fig. 1. Ubiquitylation is a multistep reaction. (Lorenz S, et al., 2013)
Reference:
- Lorenz S, Cantor A J, Rape M, et al. REVIEW Macromolecular juggling by ubiquitylation[J]. 2013.


