Microtubules
About Microtubules
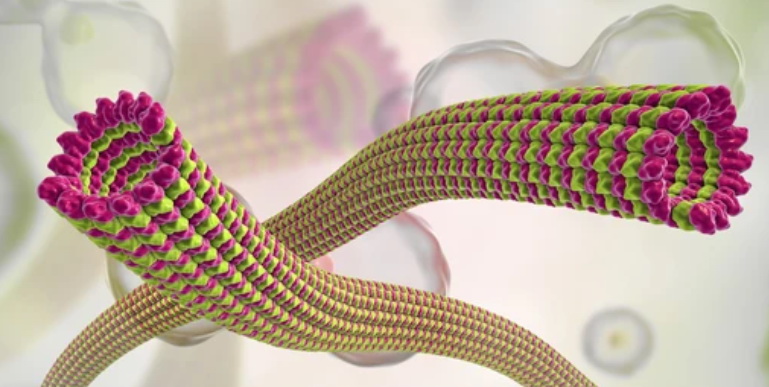
Microtubules are cylindrical structures that form part of the cytoskeleton, a network of protein filaments within cells. They have a hollow tube-like structure, composed primarily of the protein tubulin, and play crucial roles in a variety of cellular processes.
Microtubules are involved in maintaining cell shape and providing structural support to cells. They act as tracks along which organelles and vesicles can move within the cell, facilitating intracellular transport. They also play a role in cell division by forming the spindle apparatus, which helps to separate chromosomes during mitosis and meiosis.
In addition to their structural roles, microtubules are involved in cell motility. They form the core of cilia and flagella, which are hair-like structures that extend from the surface of many types of cells. Microtubules within cilia and flagella undergo a coordinated sliding motion, which allows for the movement of fluids or the entire cell.
Microtubules are dynamic structures that can undergo rapid assembly and disassembly. This dynamic behavior is regulated by various proteins, including microtubule-associated proteins (MAPs) and motor proteins. MAPs help to stabilize microtubules and regulate their interactions with other cellular components, while motor proteins use the energy from ATP hydrolysis to move along microtubules and transport cargo.
Abnormalities in microtubule function have been implicated in various diseases. For example, defects in cilia and flagella microtubules can lead to disorders called ciliopathies, which can affect multiple organs and systems in the body. Disruptions in microtubule dynamics have also been associated with neurodegenerative diseases, such as Alzheimer's and Parkinson's disease.
In conclusion, microtubules are essential components of the cytoskeleton and play diverse roles in maintaining cell structure, facilitating intracellular transport, promoting cell motility, and assisting in cell division. Their dynamic nature and involvement in various cellular processes make them crucial for the overall functioning and survival of cells. Additionally, microtubules are targets for several therapeutic interventions, making them important in medical research and the development of treatments for various diseases. Understanding the intricate mechanisms of microtubule organization and regulation is vital for advancing our knowledge of cellular biology and potentially addressing diseases associated with cytoskeletal dysfunction.
Biological Functions of Microtubules-associated Proteins
KATNA1 (Katanin p60 ATPase-containing subunit A1):
- KATNA1 is a subunit of the katanin complex, which is involved in microtubule severing and regulation of microtubule dynamics.
- It plays a role in microtubule organization, particularly in the regulation of microtubule length and dynamics during processes such as cell division, intracellular transport, and neuronal development.
- Aurora A kinase is a protein kinase that localizes to centrosomes and spindle microtubules during cell division.
- It regulates various processes during mitosis, including centrosome maturation, spindle assembly, and chromosome segregation.
- Aurora A is involved in the regulation of microtubule dynamics and organization, ensuring accurate cell division and genomic stability.
- Aurora B kinase is a component of the chromosomal passenger complex and is primarily localized to the kinetochores of chromosomes during mitosis.
- It regulates chromosome alignment, spindle checkpoint signaling, and cytokinesis during cell division.
- AURKB is involved in the phosphorylation of microtubule-associated proteins, contributing to the regulation of microtubule dynamics and the coordination of mitotic events.
- DCTN1 is a subunit of the dynactin complex, which interacts with microtubules and dynein motor proteins.
- It plays a role in the regulation of cytoplasmic dynein-mediated transport along microtubules.
- DCTN1 is involved in various intracellular processes, including organelle transport, vesicle trafficking, and the positioning of the mitotic spindle during cell division.
- DCTN2 is another subunit of the dynactin complex that interacts with microtubules and dynein.
- It is involved in the regulation of dynein-mediated motility and cargo transport along microtubules.
- DCTN2 contributes to various cellular processes, including organelle transport, vesicle trafficking, and mitotic spindle positioning.
MAPT (Microtubule-Associated Protein Tau):
- MAPT is a protein that stabilizes and promotes the assembly of microtubules in neurons.
- It is primarily expressed in the central nervous system and plays a critical role in the development and maintenance of neuronal cell shape and axonal transport.
- MAPT is associated with neurodegenerative diseases, where its abnormal aggregation is implicated in the pathogenesis of Alzheimer's disease and other tauopathies.
- STMN2 is a microtubule-destabilizing protein that regulates microtubule dynamics.
- It binds to tubulin subunits and inhibits microtubule polymerization, promoting microtubule disassembly and turnover.
- STMN2 is involved in neuronal development, axonal growth, and synaptic plasticity.
- TUBB3 is a specific isoform of beta-tubulin that is predominantly expressed in neurons.
- It is a structural component of microtubules and contributes to their assembly and stability.
- TUBB3 is involved in neuronal development, axonal guidance, and neuronal migration.
These proteins are associated with microtubules and have critical roles in regulating microtubule dynamics, organization, and functions in various cellular processes, including cell division, intracellular transport, neuronal development, and cytoskeletal dynamics.
The Application Areas of Microtubules-associated Proteins
Microtubule-associated proteins (MAPs) have diverse application areas due to their crucial roles in microtubule organization, dynamics, and cellular processes. Here are some application areas where microtubule-associated proteins are utilized:
Cancer Research and Therapy
- Microtubule-targeting agents (MTAs) that bind to microtubules and disrupt their dynamics are commonly used in cancer chemotherapy.
- MAPs, such as Tau and stathmin, are studied to understand their roles in cancer cell proliferation, metastasis, and drug resistance.
- MAPs can serve as potential targets for the development of novel anticancer drugs or as biomarkers for cancer diagnosis and prognosis.
Neurodegenerative Diseases
- Microtubule-associated protein Tau is highly relevant in neurodegenerative diseases, particularly Alzheimer's disease and other tauopathies.
- Research focuses on understanding the pathological aggregation of Tau and its impact on microtubule stability and neuronal function.
- MAPs associated with neurodegenerative diseases can serve as therapeutic targets or biomarkers for disease diagnosis and monitoring.
Neuronal Development and Regeneration
- Microtubule-associated proteins play critical roles in neuronal development, axonal guidance, and synaptic plasticity.
- They are studied in the context of neural development, neuroregeneration, and promoting axonal growth after injury.
- MAPs can be utilized in the design of biomaterials or therapeutic strategies to support neural tissue engineering and regeneration.
Drug Development and Screening
- Microtubule-associated proteins are potential targets for drug development aimed at modulating microtubule dynamics.
- Researchers investigate MAPs to identify compounds or small molecules that can regulate microtubule stability and dynamics for therapeutic purposes.
- Screening assays involving MAPs are used to discover new drugs or evaluate the efficacy of potential microtubule-targeting agents.
Cell Biology and Biotechnology
- Understanding microtubule dynamics and functions is crucial in cell biology and biotechnology research.
- Microtubule-associated proteins are used as markers to visualize and track microtubules in live cells.
- They are employed in studying intracellular transport, cell division, cell migration, and cytoskeletal dynamics in various cell types.
Biomaterials and Tissue Engineering
- Microtubule-associated proteins can be utilized in the design and development of biomaterials and scaffolds for tissue engineering applications.
- They can be incorporated into scaffolds to guide cellular processes, such as neurite outgrowth or the alignment of cells along microtubule tracks.
- Microtubule-associated proteins can enhance the functionality and organization of engineered tissues and promote tissue regeneration.
Biophysical Studies and Drug Delivery
- Microtubule-associated proteins are studied to understand the mechanical properties and behavior of microtubules.
- They are used in biophysical studies to analyze microtubule polymerization, stability, and interactions with other cellular components.
- MAPs can be utilized in drug delivery systems by exploiting their interactions with microtubules for targeted and controlled release of therapeutic agents.
These are just a few examples of the application areas of microtubule-associated proteins. Their roles in microtubule organization, dynamics, and cellular processes make them valuable targets for research, drug development, and various biomedical and biotechnological applications.
Available Resources for Microtubules-associated Proteins
Creative BioMart is a leading provider in life sciences research, delivering a diverse range of products, custom services, and resources related to microtubule-associated proteins. Within our product range, you can find recombinant proteins, cell and tissue lysates, protein pre-coupled magnetic beads, antibodies, and associated items. Furthermore, we offer customized services and an extensive array of technical resources to meet the unique needs of our clientele. Below, you'll find a compilation of molecules associated with microtubule-associated proteins. Click on a molecule/target to access our comprehensive resources:


