Monocyte/Macrophage
About Monocyte/Macrophage
Monocytes and macrophages are essential components of the immune system and play critical roles in various physiological and pathological processes. They are part of the mononuclear phagocyte system and are derived from a common precursor called the monocyte-macrophage progenitor in the bone marrow.
Monocytes are a type of white blood cell that circulates in the bloodstream. They are part of the innate immune system and act as "sentinels" by patrolling the blood vessels and tissues, ready to respond to infection or tissue damage. Monocytes are characterized by their kidney-shaped nucleus and a relatively large cell size. Main functions of monocytes:
- Phagocytosis: Monocytes can engulf and destroy pathogens, cellular debris, and foreign substances through phagocytosis. They recognize and bind to pathogens through various receptors, internalize them, and degrade them using enzymes and antimicrobial molecules.
- Antigen Presentation: Monocytes can present antigens to T cells, initiating adaptive immune responses. They process antigens from engulfed pathogens and present them on their cell surface in complex with major histocompatibility complex (MHC) molecules, thereby activating T cells and coordinating immune responses.
- Cytokine Production: Monocytes produce a wide range of cytokines, including pro-inflammatory cytokines (such as interleukin-1, interleukin-6, and tumor necrosis factor-alpha) and anti-inflammatory cytokines (such as interleukin-10). These cytokines help regulate immune responses and coordinate the recruitment and activation of other immune cells.
Macrophages are derived from monocytes and play diverse roles in tissue homeostasis, immune defense, and tissue repair. They are found in almost all tissues and organs throughout the body. Macrophages have a more differentiated morphology compared to monocytes, with a larger size and a more irregular shape. Main functions of macrophages:
- Phagocytosis and Pathogen Clearance: Macrophages are highly phagocytic cells and play a crucial role in engulfing and eliminating pathogens, cellular debris, and dead cells. They have an extensive array of receptors that recognize and bind to pathogens, allowing them to initiate phagocytosis and subsequent destruction.
- Tissue Remodeling and Repair: Macrophages contribute to tissue remodeling and repair processes. They remove apoptotic cells and debris, secrete growth factors and cytokines that promote tissue regeneration, and participate in tissue remodeling by degrading and reorganizing extracellular matrix components.
- Immune Regulation: Macrophages are involved in immune regulation by producing cytokines and chemokines that influence the activation and differentiation of other immune cells. They can promote inflammation or dampen excessive immune responses, thereby maintaining immune homeostasis.
- Antigen Presentation: Similar to monocytes, macrophages can present antigens to T cells, initiating adaptive immune responses. They capture process, and present antigens to T cells, playing a crucial role in the activation and regulation of adaptive immunity.
- Host Defense: Macrophages contribute to host defense against infections by secreting antimicrobial molecules, such as reactive oxygen species and antimicrobial peptides. They also coordinate immune responses by recruiting and activating other immune cells.
Monocytes and macrophages are dynamic and versatile cells that participate in immune surveillance, tissue homeostasis, and host defense. Their functions are tightly regulated and are essential for maintaining a balanced immune response. Understanding the roles of monocytes and macrophages is crucial for studying immune-related diseases, developing therapeutic interventions, and advancing our knowledge of immune system biology.
The Connection between Monocytes and Macrophages
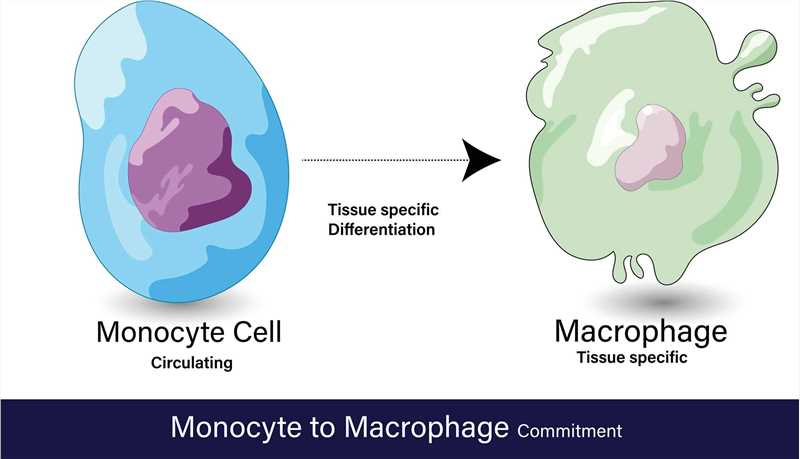
Monocytes and macrophages are closely connected as they are at different stages of the same cell lineage. Monocytes are the precursors of macrophages, and their differentiation into macrophages occurs primarily in tissues.
When monocytes are released from the bone marrow into the bloodstream, they circulate as immature cells. Upon encountering inflammatory signals or tissue damage, monocytes migrate out of the bloodstream and into tissues. Once in the tissues, monocytes undergo a series of differentiation steps and transform into mature macrophages.
The differentiation of monocytes into macrophages is influenced by various factors, including local tissue microenvironment, cytokines, and growth factors. These factors stimulate monocytes to undergo morphological and functional changes, acquiring the characteristics of tissue-resident macrophages.
Once differentiated into macrophages, they take on specialized roles depending on the tissue they reside in. For example, in the liver, they are known as Kupffer cells, while in the brain, they are called microglia. Macrophages in different tissues adapt to their specific microenvironment and perform tissue-specific functions.
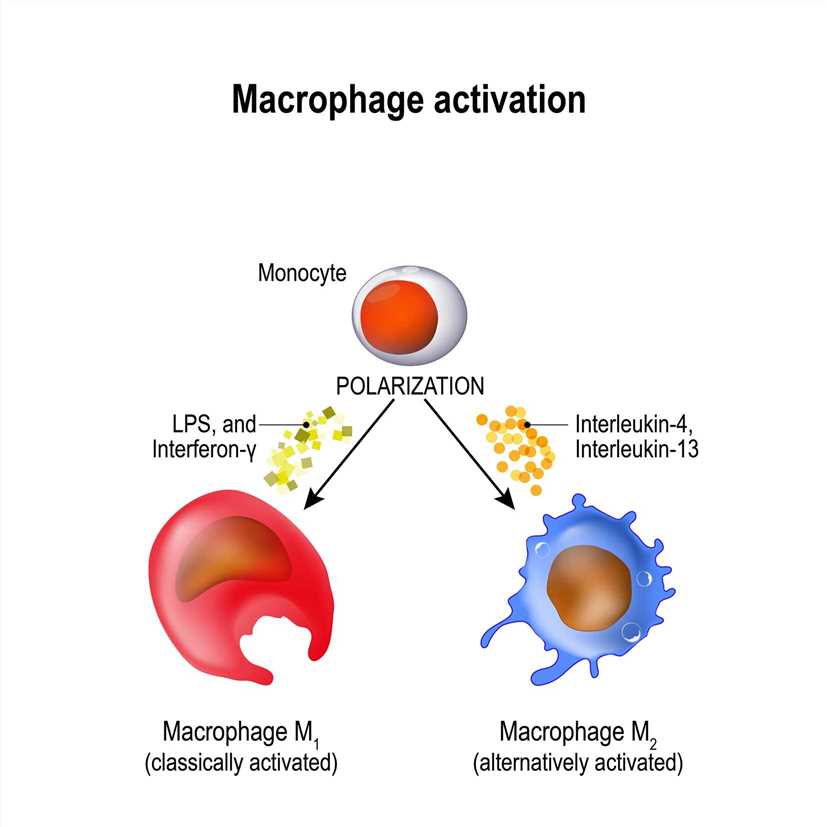
The differentiation of monocytes into macrophages is not a one-way process. Macrophages can also undergo phenotypic changes and differentiate into specialized macrophage subsets in response to specific stimuli. These subsets include M1 macrophages, which have pro-inflammatory functions, and M2 macrophages, which have anti-inflammatory and tissue repair functions. These subsets can coexist in tissues and play distinct roles in immune responses and tissue homeostasis.
In summary, monocytes and macrophages are interconnected stages of the same cell lineage. Monocytes serve as circulating precursors, and upon migration into tissues, they differentiate into tissue-resident macrophages. The plasticity of macrophages allows them to adapt to tissue-specific functions and respond to environmental cues, contributing to immune responses, tissue homeostasis, and repair processes.
Modulation of Autoimmune Diseases by Monocyte and Macrophage
Monocytes and macrophages are key players in autoimmune diseases. During the development of autoimmune diseases, pro-inflammatory M1 monocytes or macrophages can secrete various chemokines to recruit additional immune cells (i.e., T cells, B cells, neutrophils, NK cells, and NKT cells) to the affected tissues. Then, monocyte or macrophage can activate these cells via the secretion of various pro-inflammatory cytokines (i.e., IL-1β, IL-6, IL-12, IL-23, IFN-γ, and TNF-α) or through direct cell-cell contact (antigen presentation: MHC, co-stimulation: CD80, CD86 and CD40, and adhesion molecules: CD169). In addition, monocytes or macrophages can also exert direct tissue injury functions by producing matrix metalloproteinases (MMPs) and reactive oxygen species (ROS). Consequently, the activation of monocytes or macrophages and other immune cells synergistically leads to tissue damage. On the other hand, M2 monocytes or macrophages mediate immunosuppressive or tissue-repairing effects during this process, mainly by producing cytokines (i.e., IL-10 and TGF-β) and growth factors (i.e., PDGF and VEGF). M2 monocyte or macrophage can also secrete various pro-fibrotic factors, such as TGF-β, PDGF, and VEGF, to activate myofibroblasts in certain tissues, leading to extracellular matrix deposition and fibrosis generation (i.e., cases in PBC and SSc).
 Fig.3 Modulation of autoimmune diseases by monocyte and macrophage. (Wen-Tao Ma, et al., 2019)
Fig.3 Modulation of autoimmune diseases by monocyte and macrophage. (Wen-Tao Ma, et al., 2019)
Available Resources for Monocyte/Macrophage
Creative BioMart is a company dedicated to providing products related to monocytes/macrophages. We have a wide range of products, including recombinant proteins and many other types. At the same time, we provide personalized services according to the specific needs of our customers.
In addition, we provide comprehensive resources related to monocytes/macrophages. These resources cover many aspects of monocytes/macrophages, including involved pathways, protein functions, interacting proteins, related articles, overviews of research areas, and in-depth discussions of a variety of related topics. We are committed to providing researchers with accurate and useful information to help them further their research in the field of monocytes/macrophages.
We strive to provide high-quality and reliable products and services in both research and applications. We work closely with our customers to understand their needs and provide customized solutions according to specific requirements. Our team consists of experienced specialists with in-depth knowledge and research experience in the field of monocytes/macrophages. Whether it is experiment design, technical consultation, or experiment execution, we can provide professional support and advice.
If you have any questions, requirements, or cooperation intentions, please feel free to contact us. We very much look forward to working with you and helping you achieve research and commercial success.
References:
- Ma W T, Gao F, Gu K, et al. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review[J]. Frontiers in Immunology, 2019, 10:1140-. DOI:10.3389/fimmu.2019.01140.
- Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1. Published 2014 Jan 7. doi:10.1186/2050-7771-2-1
- Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation[J]. Frontiers in Immunology, 2014, 5:1-22. DOI:10.3389/fimmu.2014.00514.
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014 Jun;14(6):392-404. doi: 10.1038/nri3671. PMID: 24854589.


