Platelet-Derived Growth Factor (PDGF) Family
Available Resources for the Study of PDGF Family
Creative BioMart is dedicated to advancing research in the field of the PDGF family. Our mission is to offer researchers the most cutting-edge tools and knowledge about PDGF receptors and ligands
- We provide a diverse range of products, including recombinant proteins, pre-coupled magnetic beads, cell and tissue lysates, and more, all of which are essential for gaining a comprehensive understanding of the functions and mechanisms of the PDGF family.
- Our team of experts boasts extensive experience in PDGF family research and is committed to developing tailored solutions to address the specific requirements of researchers.
- Furthermore, we offer extensive resources such as involved pathways, protein function, interacting proteins, and other valuable information to support research endeavors and amplify their impact.
Our Featured Products
About PDGF Family
The Platelet-Derived Growth Factor (PDGF) family is a group of growth factors and their corresponding receptors that play crucial roles in various physiological and pathological processes. The PDGF family consists of four members: PDGF-A, PDGF-B, PDGF-C, and PDGF-D, along with two receptor isoforms: PDGFR-α and PDGFR-β.
PDGF Ligands
- PDGF-A and PDGF-B: These are the classical PDGF ligands and are secreted as homodimers (AA and BB) or heterodimers (AB). They are primarily involved in paracrine signaling and are derived from platelets, activated endothelial cells, smooth muscle cells, and other cell types. PDGF-A and PDGF-B play crucial roles in promoting cell proliferation, migration, and survival, especially in mesenchymal cells.
- PDGF-C and PDGF-D: These are relatively newer members of the PDGF family. PDGF-C can form homodimers (CC) or heterodimers (CD) with PDGF-D. PDGF-C and PDGF-D are secreted as latent factors and require proteolytic cleavage for activation. They are involved in autocrine and paracrine signaling and have been implicated in tissue remodeling, wound healing, and fibrotic diseases.
PDGF Receptors
- PDGFR-α: This receptor isoform preferentially binds to PDGF-A and PDGF-C ligands. It is primarily expressed on mesenchymal cells, including fibroblasts, smooth muscle cells, pericytes, and glial cells. PDGFR-α plays a crucial role in development, tissue repair, and fibrosis.
- PDGFR-β: This receptor isoform has a higher affinity for PDGF-B and PDGF-D ligands. It is expressed on cells of both mesenchymal and hematopoietic origins, including fibroblasts, smooth muscle cells, pericytes, osteoblasts, and immune cells such as macrophages. PDGFR-β is involved in processes such as angiogenesis, hematopoiesis, and immune responses.
The binding of PDGF ligands to their respective receptors leads to receptor dimerization and autophosphorylation of specific tyrosine residues within the intracellular domain. This activation triggers downstream signaling pathways, including the Ras-MAPK, PI3K-Akt, and PLCγ pathways. These pathways regulate various cellular processes, including cell growth, proliferation, migration, and differentiation.
The PDGF family and its receptors are crucial for embryonic development, tissue repair, and maintenance of tissue homeostasis. Dysregulation of PDGF signaling is associated with several diseases, including cancer, fibrosis, and vascular disorders. Therefore, targeting PDGF ligands or receptors has been explored as a potential therapeutic strategy in the treatment of these diseases.
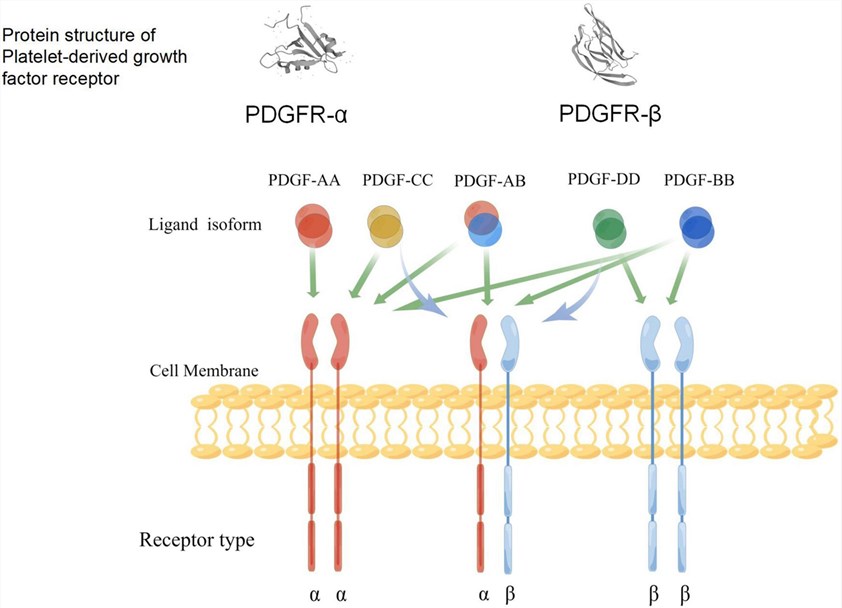 Fig.1 A schematic diagram of the specific binding of PDGF ligand to the receptors, and the structure of its receptors. (Li D, et al., 2022)
Fig.1 A schematic diagram of the specific binding of PDGF ligand to the receptors, and the structure of its receptors. (Li D, et al., 2022)
PDGF Family in Disease
The PDGF family has been implicated in various diseases due to its role in promoting cell growth, proliferation, and survival. Dysregulation of PDGF signaling can contribute to the development and progression of several pathological conditions. Here are some examples of diseases where the PDGF family has been implicated:
- Cancer: PDGF signaling has been associated with several types of cancer, including glioblastoma, sarcoma, lung cancer, and ovarian cancer. In cancer, PDGF ligands can be produced by tumor cells or within the tumor microenvironment, leading to autocrine or paracrine activation of PDGF receptors. This results in enhanced tumor cell proliferation, angiogenesis, and recruitment of stromal cells. Targeting PDGF signaling has been explored as a potential therapeutic strategy in cancer treatment.
- Fibrosis: Excessive activation of PDGF signaling has been implicated in fibrotic diseases, such as pulmonary fibrosis, liver fibrosis, and kidney fibrosis. During fibrosis, PDGF ligands are released in response to tissue injury, leading to the activation of PDGF receptors on fibroblasts and other mesenchymal cells. This promotes the proliferation and activation of fibroblasts, leading to the excessive deposition of extracellular matrix and tissue scarring. Inhibition of PDGF signaling has shown promise in attenuating fibrosis in preclinical and clinical studies.
- Vascular Diseases: Dysregulation of PDGF signaling can contribute to various vascular disorders, including atherosclerosis, restenosis (narrowing of blood vessels after angioplasty), and pulmonary arterial hypertension. PDGF signaling can promote smooth muscle cell proliferation and migration, leading to the thickening of blood vessel walls and the development of vascular lesions. Inhibiting PDGF signaling has been explored as a potential therapeutic approach in these vascular diseases.
- Retinal Diseases: PDGF signaling has been implicated in retinal diseases, particularly proliferative retinopathies such as diabetic retinopathy and retinopathy of prematurity. In these diseases, the abnormal growth of blood vessels in the retina can lead to vision loss. PDGF signaling is involved in the recruitment and activation of pericytes, which play a role in stabilizing blood vessels. Modulating PDGF signaling has been investigated as a potential therapeutic strategy to inhibit abnormal blood vessel growth and preserve retinal function.
- Skin Disorders: PDGF signaling has been implicated in various skin disorders, including psoriasis, keloids, and wound healing abnormalities. In psoriasis, abnormal activation of PDGF receptors on keratinocytes and immune cells contributes to the inflammatory response and excessive cell proliferation. In keloids, elevated PDGF expression and signaling contribute to the excessive deposition of collagen and fibrotic tissue. Modulating PDGF signaling has been explored as a potential therapeutic approach in these skin disorders.
These are just a few examples of the involvement of the PDGF family in disease processes. The dysregulation of PDGF signaling can have varied effects depending on the specific disease context, but targeting PDGF ligands or receptors has emerged as a potential therapeutic strategy in several diseases. Further research is needed to fully understand the mechanisms underlying PDGF-mediated diseases and to develop effective therapeutic interventions.
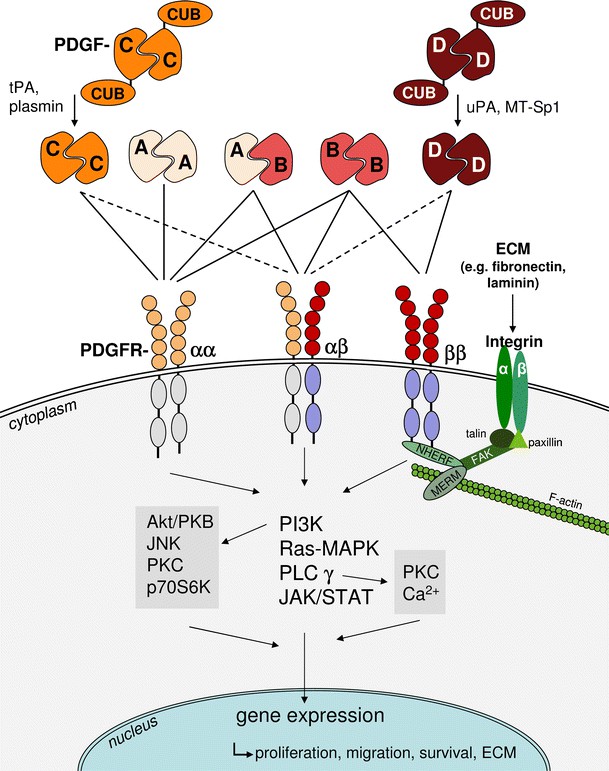 Fig.2 Simplified scheme of the main molecules involved in PDGF–PDGFR interactions. (Ostendorf, et al., 2014)
Fig.2 Simplified scheme of the main molecules involved in PDGF–PDGFR interactions. (Ostendorf, et al., 2014)
We are committed to helping you achieve your scientific goals and make meaningful contributions to research on the roles of the various components of the PDGF family and their role in disease. Contact us today to learn more about our products and resources.
References:
- Li D, Huang L T, Zhang C, et al. Insights Into the Role of Platelet-Derived Growth Factors: Implications for Parkinson's Disease Pathogenesis and Treatment[J]. Frontiers in Aging Neuroscience, 2022, 14: 890509.
- Ostendorf, Boor, VanRoeyen, et al. Platelet-derived growth factors (PDGFs) in glomerular and tubulointerstitial fibrosis[J].[2024-03-04].
- Ostendorf, T., Eitner, F. & Floege, J. The PDGF family in renal fibrosis. Pediatr Nephrol 27, 1041–1050 (2012).


