Wnt Family
Available Resources for The Study of Wnt Family
Creative BioMart is committed to supporting research on the Wnt family. We continually update our portfolio and resources to provide researchers with tools and information about the Wnt family (e.g., Wnt inhibitors, Wnt intracellular signaling, Wnt ligands (Wnts), Wnt receptors, Wnt signaling modulators). To advance research on the complexity of the Wnt signaling pathway to help develop safe and effective therapeutic interventions.
- Our broad product portfolio includes recombinant proteins, etc., which play a key role in elucidating the functions and mechanisms of various players in the Wnt family.
- We have a team of experienced experts with deep knowledge in the study of the Wnt family, and we are committed to providing tailored solutions to meet the unique requirements of each researcher.
- In addition, we provide comprehensive resource support including involved pathways, protein function, interacting proteins, and other valuable information. Ultimately, our goal is to increase the impact of research efforts.
Our Featured Products
About Wnt Family
Here's a comprehensive introduction to various aspects of the Wnt family, including Wnt inhibitors, Wnt intracellular signaling, Wnt ligands (Wnts), Wnt receptors, and Wnt signaling modulators:
Wnt Inhibitors
- Wnt inhibitors are molecules that regulate or dampen the activity of the Wnt signaling pathway. They act to antagonize or inhibit Wnt signaling. Examples of Wnt inhibitors include:
- Dickkopf (DKK) proteins: DKK proteins bind to the Wnt co-receptors LRP5/6, preventing the interaction between Wnt ligands and the receptors, thereby inhibiting Wnt signaling.
- Secreted Frizzled-Related Proteins (sFRPs): sFRPs bind to Wnt ligands and FZD receptors, interfering with the Wnt-FZD interaction and inhibiting Wnt signaling.
- Wnt Inhibitory Factor 1 (WIF-1): WIF-1 binds to Wnt ligands, preventing their interaction with FZD receptors and inhibiting Wnt signaling.
Wnt Intracellular Signaling
The Wnt intracellular signaling pathways refer to the series of events that occur inside the cell upon Wnt ligand binding to its receptors. The two main Wnt signaling pathways are:
- Canonical Wnt/β-catenin pathway: In the absence of Wnt ligands, a destruction complex degrades β-catenin, preventing its accumulation in the nucleus. Upon Wnt ligand binding to FZD receptors and LRP5/6 co-receptors, the destruction complex is inhibited, allowing β-catenin to accumulate and translocate into the nucleus. There, it interacts with TCF/LEF transcription factors, activating the transcription of target genes.
- Non-canonical Wnt pathways: Non-canonical pathways include the Wnt/Ca2+ pathway and the planar cell polarity pathway. These pathways do not involve the stabilization of β-catenin. Instead, they activate different intracellular signaling cascades, leading to various cellular responses such as changes in intracellular calcium levels, cytoskeletal rearrangements, and cell polarity.
Wnt Ligands (Wnts)
- Wnt ligands are a group of secreted glycoproteins that act as signaling molecules in the Wnt pathway. They bind to cell surface receptors to initiate intracellular signaling. The Wnt family consists of 19 members in humans (WNT1-WNT19), each with specific expression patterns and functions. Different Wnt ligands can activate different branches of the Wnt pathway and elicit distinct cellular responses.
Wnt Receptors
Wnt ligands bind to cell surface receptors to initiate intracellular signaling. The primary receptors involved in Wnt signaling are:
- Frizzled (FZD) receptors: FZD receptors are seven-transmembrane domain receptors that bind to Wnt ligands. They transmit the Wnt signal into the cell and activate downstream signaling pathways.
- Co-receptors: Low-density lipoprotein receptor-related protein 5/6 (LRP5/6) are co-receptors that interact with FZD receptors and contribute to Wnt signaling activation. They play a crucial role in the canonical Wnt/β-catenin pathway.
Wnt Signaling Modulators
Wnt signaling modulators are proteins that regulate the activity and output of the Wnt signaling pathway. They can enhance (agonists) or inhibit (antagonists) Wnt signaling to fine-tune cellular responses. Examples of Wnt signaling modulators include:
- R-spondins (RSPO): RSPOs enhance Wnt signaling by inhibiting the degradation of LRP5/6 via the E3 ubiquitin ligases RNF43 and ZNRF3.
- Norrin: Norrin is an agonist that activates Wnt signaling, particularly in retinal and vascular development.
- Axin: Axin is a scaffolding protein that regulates the activity of the destruction complex in the canonical pathway, thereby modulating β-catenin levels and Wnt signaling strength.
Understanding the interplay between Wnt inhibitors, intracellular signaling, ligands, receptors, and signaling modulators is essential to comprehending the complex regulation and diverse functions of the Wnt family in development, homeostasis, and disease.
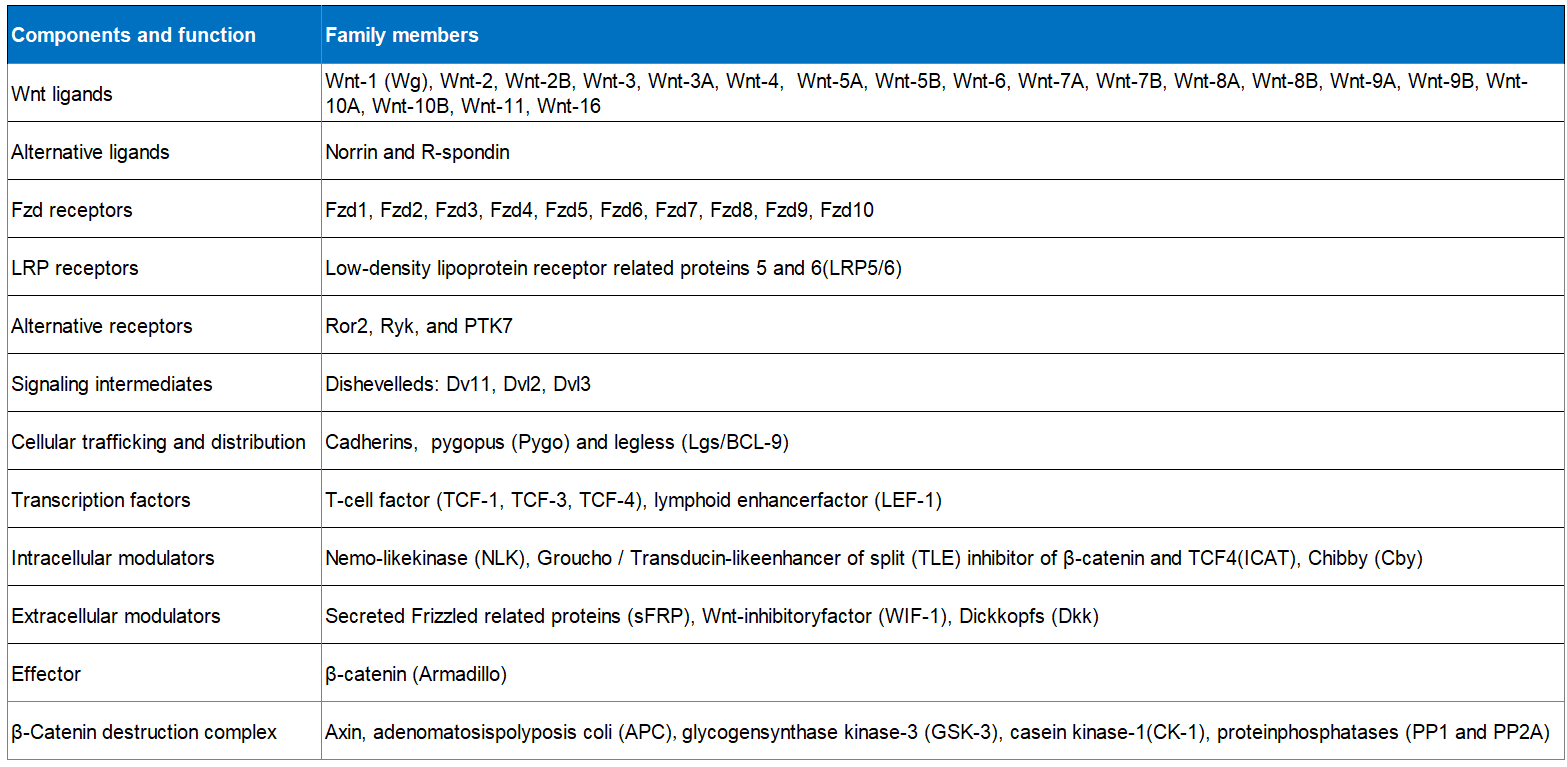
Table.1 The various ligands, receptors, components, and Wnt family members involved in the Wnt signaling pathway. (Newmire, et al., 2015)
Importance of the Wnt Signaling Pathway in Disease
The Wnt signaling pathway plays a crucial role in various diseases, and its dysregulation has been implicated in the pathogenesis of numerous disorders. Understanding the importance of the Wnt signaling pathway in disease can lead to the development of potential therapeutic strategies. Here are some key points highlighting the significance of the Wnt signaling pathway in disease and its potential therapeutic applications:
- Cancer: Dysregulated Wnt signaling is a hallmark of many cancers. Aberrant activation of the canonical Wnt/β-catenin pathway, often through mutations in key pathway components, promotes tumor initiation, progression, and metastasis. Targeting the Wnt pathway presents an opportunity for developing novel cancer therapeutics.
- Neurological Disorders: Disruptions in Wnt signaling have been implicated in neurodegenerative diseases such as Alzheimer's and Parkinson's disease. Modulating Wnt signaling components may hold potential for neuroprotection and promoting neuronal survival and regeneration.
- Skeletal Disorders: The Wnt signaling pathway is essential for skeletal development and homeostasis. Mutations in Wnt pathway components contribute to skeletal disorders such as osteoporosis, osteoarthritis, and bone metastasis. Therapeutic interventions targeting Wnt signaling could enhance bone formation, inhibit bone resorption, and potentially treat skeletal disorders.
- Cardiovascular Diseases: Wnt signaling is involved in cardiovascular development, angiogenesis, and cardiac remodeling. Dysregulated Wnt signaling has been associated with heart failure, atherosclerosis, and cardiac fibrosis. Modulating the Wnt pathway may offer therapeutic approaches for managing cardiovascular diseases.
It's important to note that while targeting the Wnt signaling pathway shows great promise, further research is needed to fully understand its complexity and develop safe and effective therapeutic interventions. Nonetheless, the therapeutic potential of modulating the Wnt pathway in disease holds significant promise for improving patient outcomes in various fields of medicine.
We are committed to helping you achieve your scientific goals and make meaningful contributions to research on the roles of the various components of the Wnt family and their role in disease. Contact us today to learn more about our products and resources.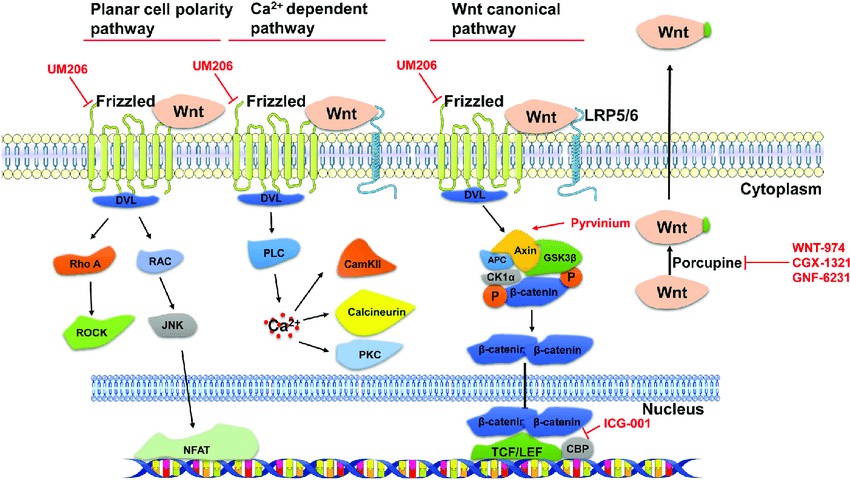 Fig.1 Wnt signaling pathways and the intervention targets of Wnt pathway inhibitors. (Fu WB, et al., 2018)
Fig.1 Wnt signaling pathways and the intervention targets of Wnt pathway inhibitors. (Fu WB, et al., 2018)
References:
- Newmire, Dan, & Darryn S. Willoughby. "Wnt and β-Catenin Signaling and Skeletal Muscle Myogenesis in Response to Muscle Damage and Resistance Exercise and Training." International Journal of Kinesiology and Sports Science [Online], 3.4 (2015): 40-49. Web. 21 Feb. 2024.
- Fu WB, Wang WE, Zeng CY. Wnt signaling pathways in myocardial infarction and the therapeutic effects of Wnt pathway inhibitors. Acta Pharmacol Sin. 2019;40(1):9-12.


