Protocol of Preparation of the Total Protein Samples
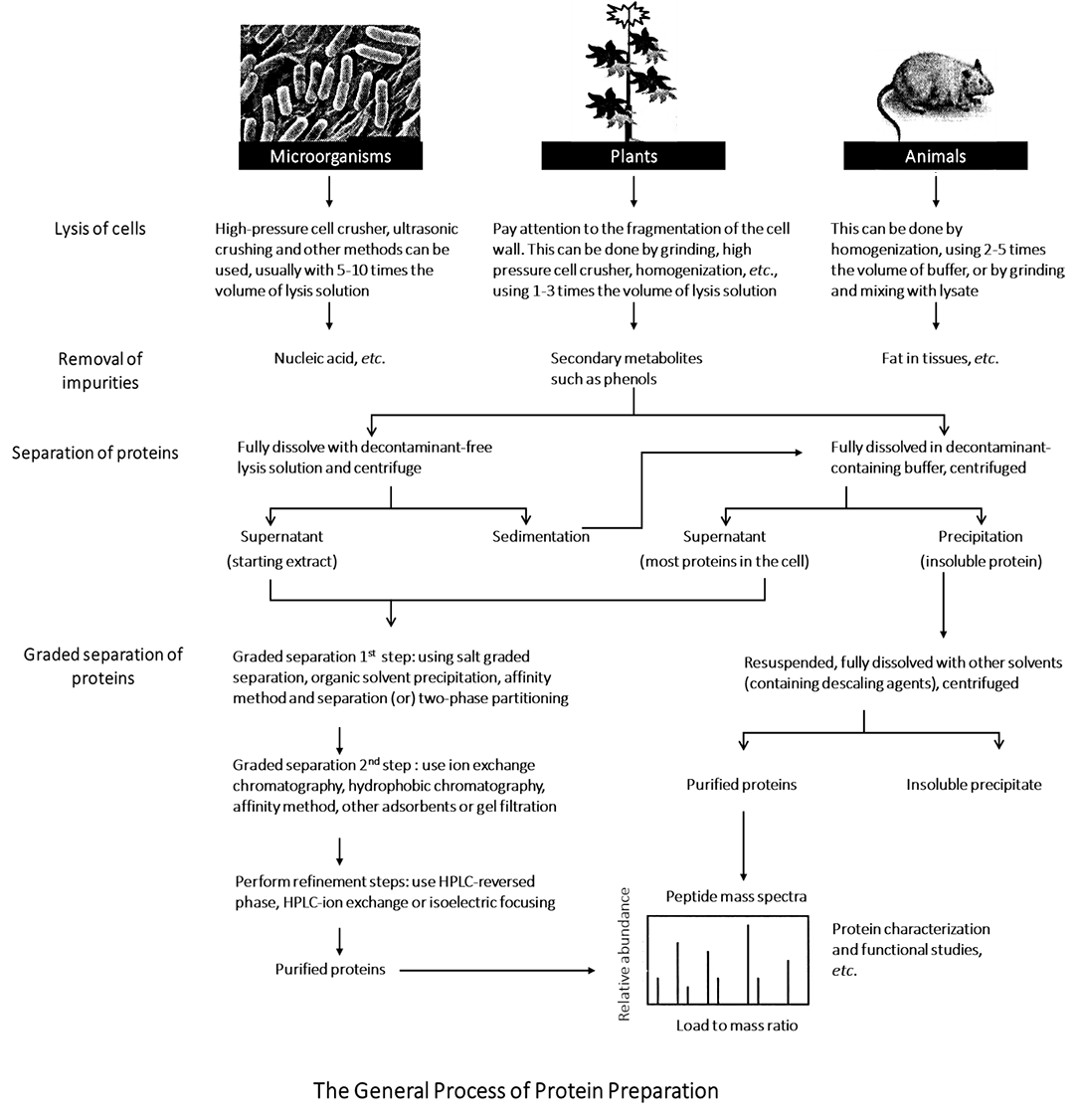
Proteomics mainly studies the expression of cell proteins and obtains information such as the expression profile of protein. The research object of proteomics is large range protein rather than single protein. Therefore, the research program should be able to obtain proteins as abundant as possible. There are many kinds of proteins in the organism, with huge differences in expression abundance as well as physical and chemical properties, making it difficult to prepare total proteins. The preparation of total protein samples is actually a relative concept. To obtain relatively abundant proteins, it is necessary to prepare the samples step by step according to the basic sample preparation principles of specific experimental operations. The process mainly involves cell fragmentation, protein precipitation and protein dissolution.
At present, plenty of total protein preparation methods have been developed for different biomaterials, tissues, cells and specific target proteins. An experimental plan can be formulated referring to the literature and experiences to prepare high-quality protein samples. In general, the following principles should be followed in scheme formulation.
1. The applied methods to obtain abundant target proteins should be as simple as possible. Remove as much as possible the high abundance or irrelevant proteins that interfere, so as to reduce protein loss while ensuring the detection effect of proteins to be studied.
2. All protein samples to be analyzed should be in a dissolved state (including most hydrophobic proteins), and the preparation method should be repeatable.
3. Minimize the operations that may modify the protein in the process of protein extraction. For example, the sample containing urea is not supposed to be stored at 37 ° C, which will convert the urea into isocyanate that can modify the protein.
4. The specific requirements of subsequent experiments should be considered during the scheme formulation. If the isoelectric focusing separation is used subsequently, it is necessary to reduce the ion concentration, which requires attention to controlling the ion concentration during sample preparation or desalting after sample preparation; If the subsequent experiment is two-dimensional liquid chromatography (PF-2D) separation, it should be noted that ionic detergent should not be used in sample preparation.
Master the general principle of protein sample preparation, and design the experimental process of total protein sample preparation in combination with the experimental purpose.
Master the preparation scheme of total protein samples for different properties, tissues and organs.
Master the applications of the main reagents in the experiment, and be able to select the appropriate reagents for protein sample preparation.
Proteins exist in complex mixtures in tissues or cells. Each type of cell contains thousands of different proteins. Protein separation, purification and characterization are important parts of biological research. Presently, there is no single or a set of ready-made methods that can extract any kind of protein from the complex mixture of proteins. Typically, several methods are used together.
Protein sample preparation is an art. The methods range from simple buffer solution dissolution to the application of separating agent, reducing agent and detergent for complex extraction, and to more complex sequential extraction and subcellular separation. Although there are many methods, in general, the preparation of total protein samples mainly includes three steps: cell disruption, protein precipitation and protein lysis.
1. Cell Disruption*1
According to the degree of cell disruption, it can be divided into mild disruption methods (Table 1-1-1) and strong disruption methods (Table 1-1-2). According to the disruption method, it can be divided into the physical disruption method and the chemical method. Generally speaking, the physical disruption method is a relatively strong disruption method, while the chemical method is the mild one.
Table 1-1-1 Mild Cell Disruption Methods
| Methods | Applicable objects | Operations |
|---|---|---|
| Osmotic shock method*2 | Blood corpuscle Tissue culture cells |
Cells are suspended in osmotic lysate solution, and cell contents are released after cell swelling. |
| Freeze-thaw method | Bacterial cells Tissue culture cells |
Use liquid nitrogen to quickly and repeatedly freeze and thaw cells to break cell membranes. |
| Cell lysis buffer | Tissue culture cells | Suspend the cells in the lysate containing detergent to lyse the cells. |
| Enzymatic lysis*3 | Plant cells Bacterial cells Fungal cells |
Cells are suspended in isotonic solution containing specific enzymes, and the cell wall is removed by enzymolysis to split cells. |
Table 1-1-2 Strong Cell Disruption Methods
| Methods | Applicable objects | Operations |
|---|---|---|
| Ultrasonic method*4 | Suspension cell | The ultrasonic instrument is used to treat suspended cells, and the shear force generated by ultrasonic is used to break cells. |
| Grinding method*5 | Solid tissue Microbial cell |
The tissues or cells were frozen in liquid nitrogen and ground into powder in a mortar. |
| Homogenate method | Solid tissue Bacterial suspension |
Cut the tissue into blocks, add cold homogenate containing protease inhibitor, filter or centrifugate for collection. |
| French press method*6 | Microbial cell Cell wall containing cells |
The cell suspension is placed in the pre cooling pressure cup, and the high pressure is used to make the cells pass through the pores, generate shear force to break the cells, and collect the extrudate. |
2. Protein Precipitation
The precipitation of protein molecules from solution by agglutination is called protein precipitation. The stability of hydrophilic colloidal particles formed by proteins is affected by two factors - the hydration layer on the particle surface and the electric charge. In the absence of additional conditions, the protein particles do not agglutinate with each other. However, when these two stable factors are removed (such as adjusting the pH of the solution to the isoelectric point and adding dehydrating agent), the protein is easy to agglutinate.
If the pH of the protein solution is adjusted to the isoelectric point, the protein molecules are in an isoelectric state. However, although the mutual repulsion of the same charges between molecules disappears, there is also a hydration membrane for protection, which generally prevents agglutination. If a certain dehydrating agent is added to remove the hydration membrane of the protein molecules, the protein molecules will agglutinate each other and precipitate. On the contrary, if the protein is dehydrated first, and then the pH is adjusted to the isoelectric point, the protein can also be precipitated.
The method of protein precipitation can also be used as a method of protein sample concentration to obtain high concentration protein solution. The method of protein precipitation is shown in Table 1-1-3.
Table 1-1-3 Methods of Protein Precipitation
| Salting out method*7 | |
|---|---|
| Common reagents | Ammonium sulfate, magnesium sulfate, sodium sulfate, sodium chloride, sodium phosphate |
| Principle | High-concentration salt ions (such as SO42- and NH4+ of ammonium sulfate) have strong hydration power, which can seize the hydration layer of protein molecules and make them "dehydrate", so that protein colloidal particles coagulate and precipitate out. |
| General process | The protein was treated in a buffer containing EDTA (>50 mmol/L) to make the final concentration of protein greater than 1 mg/ml. Slowly double sulfuric acid to saturation, stir for 10-30 min, and centrifuge to collect protein. |
| Limitation | Not all proteins can be obtained, and the residual ammonium sulfate will affect the isoelectric focusing*8. |
| Organic solvent precipitation method | |
| Common reagents | TCA, acetone, ethanol, butanol |
| Principle | The organic solution reduces its activity due to the diluted concentration. As the hydration degree of the hydrophilic region decreases, the proteins begin to gather together and precipitate. |
| General process | The sample is suspended in 10% TCA solution containing 0.07% β-mercaptoethanol. Precipitate protein at - 20 °C for at least 45 min, collect protein precipitation by centrifugation, and wash with precooled acetone solution containing 0.07% β-mercaptoethanol. Freeze dry to remove residual acetone. |
| Limitation | Protein is difficult to redissolve. Protein will be degraded and modified after long-term exposure to low pH solution. |
| High salt and organic solvent combined precipitation method | |
| Common reagents | Ammonium acetate, methanol, acetone, etc. |
| General process | The protein in the sample is extracted into phenol, and the protein was precipitated the with methanol solution containing 0.1 mol/l ammonium acetate in phenol phase. After the precipitation is cleaned with methanol solution containing ammonium acetate, it should be cleaned with acetone. Remove the residual acetone by evaporation. |
| Limitation | Complex and time-consuming. |
3. Protein Lysis
To obtain the best effect in the follow-up experiment, denaturants, detergents, reducing agents, etc. will be used to denature and fully dissolve protein samples during the lysis process*9 (Table 1-1-4). The protein yield and its impact on subsequent experiments should be taken into account when selecting protein lysate. There are many kinds of protein lysate formulations, and selecting a suitable protein lysate is the first step to obtaining an ideal protein sample. The protein lysate containing SDS (sodium dodecyl sulfate) and strong ion detergent (sodium deoxycholate) can extract a large amount of protein from tissues or cells while it is easy to cause protein denaturation, which will interfere with the recognition of antigen epitopes by antibodies in subsequent immunoblotting.
Table 1-1-4 Common Reagents in Protein Lysis
| Type | Principle |
|---|---|
| Denaturant | Change the secondary structure such as hydrogen bonds to fully extend the protein, fully expose its hydrophobic center, and reduce the energy domain close to hydrophobic residues. Common reagents: urea, sulfur pulse. |
| Detergent*10 | Destroy the hydrophobic interaction between protein molecules. Resulted from the hydrophobic groups of proteins are exposed after treatment with denaturants, at least one detergent is often required to dissolve the hydrophobic groups. Common reagents: SDS, Triton X-100, NP-40, CHAPS, OBG, ASB-14. |
| Reducing agent | Break the disulfide bond between Cys residues of protein molecules to increase protein solubility. Common reagents: β- mercaptoethanol, DTT or DTE, TBP. |
1. Main Instruments and Equipment
Micropipette, high-speed centrifuge, ultracentrifuge*11, ultrasonic crusher, mortar.
2. Experimental Materials
Cultured cells, plant or animal tissues, bacterial cultures.
3. Main reagents
(1) Lysis solution*12
| Lysis solution A (applicable to the extraction of water-soluble protein) | |
| 40 mmol/L | Tris-base (pH 9. 5) |
| Lysis solution B (classic formula) | |
| 8 mol/L | Urea |
| 4 %① | CHAPS |
| 40 mmol/L | Tris-base |
| 1 % | DTT |
| 1 % | Protease inhibitor*13 |
| Lysis solution based on classical formula (C - E)*14 | |
| Lysis solution C | |
| 7 mol/L | Urea |
| 2 mol/L | Thiourea |
| 2 % - 4 % | CHAPS |
| 40 mmol/L | Tris-base |
| 1 % | DTT |
| 2 % | Pharmalyte (pH 3 - 10) |
| 1 % | Protease inhibitor |
| Lysis solution D | |
| 9.5 mol/L | Urea |
| 2 % | CHAPS |
| 1 % | Tris-base |
| 0.8 % | Pharmalyte (pH 3 - 10) |
| 5 mmol/L | Protease inhibitor |
| Lysis solution E | |
| 9.5 mol/L | Urea |
| 2 % - 4 % | CHAPS |
| 1 % | DTT |
| 2 % | Pharmalyte (pH 3 - 10) |
| 5 mmol/L | Protease inhibitor |
| Lysis solution F (applicable to membrane protein extraction) | |
| 5 mol/L | Urea |
| 2 mol/L | Thiourea |
| 2 % | SB 3-10 |
| 2 % | CHAPS |
| 1 % | DTT |
| 0.5 % | CA |
| 5 mmol/L | Protease inhibitor |
| Lysis solution G (applicable to the extraction of insoluble precipitated protein) | |
| 1 % | SDS |
| 0. 375 mol/L | Tris-HCl (pH 8.8) |
| 50 mmol/L | DTT |
| 25% (volume) | Glycerol |
| Lysis solution H*15(applicable to the PF-2D*16) | |
| 7.5 mol/L | Urea |
| 2.5 mol/L | Thiourea |
| 12.5 % | Glycerol |
| 62.5 mmol/L | Tris-HCl |
| 2. 5 % | n-Octyl-β-D-Glucopyranoside |
| 6.25 mmol/L | Tris (2-carboxyethyl) phosphine TCEP |
| 1.25 mmol/L | Protease inhibitor |
(2) Protease inhibitor
In the process of tissue cell fragmentation, the addition of diisopropyl fluorophosphate (DFP) can inhibit or slow down autolysis. The addition of iodoacetic acid can inhibit the activity of proteolytic enzymes that require sulfhydryl groups in active centers. The activity of proteolytic enzyme can also be eliminated by adding benzene sulfonyl fluoride (PMSF), which can be added according to the actual conditions in the experiment (Table 1-1-5).
Table 1-1-5 Broad-spectrum Protease Inhibitor Mixture
| Component | Final concentration |
|---|---|
| PMSF | 35 μg/mL(1 mmol/L) |
| EDTA | 0.3 mg/mL (1 mmol/L) |
| Pepstatin | 0.7 μg/mL |
| Leupeptin | 0.5 μg/mL |
Example 1. Bacterial Cell Lysis
(1) Add 2.0 mL of lysate suspension cells to 0.5 mL of bacterial cell precipitation. Violent vortex oscillation, ice bath ultrasonic suspension of cells. Suspend cells twice with ultrasound for 30 s each time.
(2) Centrifuge the lysate at 18 °C *17,20 000 g for 60 min.
(3) Collect the supernatant, and long-term store it separately at - 80 °C (recommended) or - 20 °C.
Example 2. Tissue Homogenate Method
(1) Obtain materials.
(2) Grind the sample into powder with mortar under liquid nitrogen freezing condition, add 0.5 mL of lysis solution per gram of sample, and homogenize with tissue homogenizer for 30 s.
(3) 15 °C, 10 000 g centrifuge the tissue suspension for 10 min.
(4) 4 °C, 150 000 g ultracentrifuge the supernatant for 45 min.
(5) Carefully avoid the floating lipid layer on the upper layer, suck out the centrifuged supernatant, and centrifugate it again at 6 °C for 50 min.
(6) Take the supernatant of centrifugation. Quantitative determination by Bradford method, stored at -75 ° C after split packing.
Example 3. TCA/acetone Precipitation *18
(1) Take fresh and tender plants *19. Put the sample into the precooling mortar, add liquid nitrogen and fully grind them to powder.
(2) Add 3 times the volume of the extraction buffer [acetone solution containing 10% trichloroacetic acid (TCA) and 0.07% β-mercaptoethanol (DTT can be used as a substitute)] into a 1.5 mL centrifuge tube, add the well-ground sample powder into the centrifuge tube, mix well, and then stay overnight at - 20 °C.
(3) 4 °C, 40 000 g, centrifuge for 1 h and discard the supernatant.
(4) Resuspend the precipitate with the precooled acetone with the same volume (containing 0.07% β-mercaptoethanol) at 4 °C, 40 000 g, centrifuged for 1 h (repeatable).
(5) Vacuum dry precipitation.
(6) Fully dissolved the precipitate with the smallest volume of lysis solution, and use vortex oscillation to assist dissolution if necessary.
(7) Centrifuge the solution for 1 h*20 at 15 °C, 40 000 g, and the supernatant is the desired protein sample, which can be separately packed at - 80 °C for standby. For temporary storage, stored it at 4 °C.
Example 4. Grading Extraction Method of Plant Tissue
(1) Take frozen plant tissue, add lysis solution A, and homogenize in liquid nitrogen bath.
(2) Centrifuge at 4 ° C, 40 000 g, for 1 h, and collect the supernatant as component I *21.
(3) Fully dissolve the precipitate with the minimum volume of lysis solution B*22, which can be dissolved by vortex oscillation.
(4) Centrifuge at 15 ° C, 40 000 g, for 1 h, and collect the supernatant as component II*23.
(5) Fully dissolve the precipitate with the smallest volume of lysis solution F, which can be dissolved by vortex oscillation.
(6) Centrifuge at 15 ° C, 40 000 g, for 1 h, and collect the supernatant as component III*24.
(7) The precipitate is fully dissolved with the smallest volume of lysis solution G, which can be dissolved by vortex oscillation.
(8) Centrifuge at 15 ° C, 40 000 g, for 1 h, and collect the supernatant as component IV*25.
(9) Store protein samples at - 80 ° C or - 20 ° C*26.
1. Sample preparation is the start of the experiment and the basis of the whole experiment. Proper sample preparation method is an important part of the whole experiment, which should be given high attention.
2. During the preparation of protein samples, problems of protein dissolution are usually unavoidable, especially when extracting membrane proteins. Hydrophobic proteins can be extracted by sequential extraction protocol and treated with different surfactants.
3. In the process of protein extraction, attention should be paid to the protection of proteins. Some proteins are easy to be degraded, so protease inhibitors and reducing agents can be added and the operation should be kept at low temperature as far as possible.
*1 In order to avoid the proteases and other enzymes that cause protein modification released during cell fragmentation, protease inhibitors should be added to the experiment and kept at a low temperature during the operation.
*2 Very mild disruption method, which can be used for further subcellular separation.
*3 For cells that are not easily broken or have hard cell walls.
*4 Intermittent treatment is required to reduce the heat and foam generated. Sample treatment should be carried out in the ice bath.
*5 Aluminum oxide or sand can be added to facilitate grinding.
*6 French press cell.
*7 Typically, under low salt concentration, the solubility of protein increases with the increase of salt concentration is called salt dissolution. When the salt concentration continues to increase, the solubility of the protein decreases in varying degrees and separates successively, which is called salting out.
*8 After the protein is separated by salting out precipitation, the salt in the protein is commonly removed by dialysis. In addition, glucose gel G-25 or G-50 column chromatography can also be used for desalination.
*9 Under the condition of combination of denaturant and surfactant, the denatured protein can be expanded more completely and dissolved more thoroughly by adding reductant.
*10 Pay attention to their ionic characteristics when using detergents, such as anionic detergents (SDS), non-ionic detergents (Triton X-100, NP-40), and zwitterionic detergents (CHAPS, OBG, ASB-14).
*11 It is generally accepted that a centrifuge with a speed of 10 000-25 000 r/min is called a high-speed centrifuge. Centrifuges with speeds over 25,000 r/min and centrifugal forces greater than 89,000 g are called ultracentrifuges. Currently, the maximum speed of ultracentrifuges can reach 100,000 r/min with a centrifugal force of more than 500,000g.
*12 Lysis solution is generally freshly prepared or stored at - 20 °C for standby after sub-packaging.
*13 Proteinase inhibitors (a cocktail of protease inhibitors) and DTT are usually added before use.
*14 Lysis solution C-E can be used for sequential Extraction.
*15 Subsequent experimental methods usually require special requirements for protein sample preparation. If PF-2D separation is required after protein extraction, ionic detergents and substances with high sulfonation or sodium ions should be avoided during protein lysis.
*16 PF-2D, ProteomeLabTM PF-2D, Beckman Coulter, two-dimensional liquid chromatography protein separation system.
*17 In protein extraction operation, it is generally necessary to maintain low temperature to reduce the activity of protease and ensure the integrity of protein. However, 15~18 ° C can be used to prevent the precipitation of lysate during centrifugation and dissolution after adding lysis solution, so as to obtain a better dissolution effect.
*18 It can be used to extract total protein from plant leaves.
*19 The cellulose content of plant tissue is relatively high. Generally, samples should be prepared from parts with low cellulose content, such as tender leaves, young roots and seeds.
*20 Centrifuge until there is no precipitation. This process may require multiple and longer centrifugations.
*21 Component I is mainly water-soluble protein.
*22 In this step, any lysis solution in the classical formula or the lysis solution applicable to specific experimental materials can be added.
*23 Component II is mainly the protein in the extraction process, and the solubility of protein is between water-soluble and insoluble membrane protein.
*24 Component III is mainly membrane protein.
*25 Component IV is mainly insoluble protein.
*26 As the protein is easy to degrade, it should not be stored for a long time and should be stored at - 80 °C (recommended) or - 20 °C.
* ① Add 4g reagent to every 100mL solution, i.e., 40g/L, which is usually expressed as 4%. The rest is the same.


