Recently an international team led by Professor Savvas Savvides at the VIB-UGent Inflammatory Research Center revealed the key molecular mechanisms of autoimmune and inflammatory diseases including psoriasis, rheumatoid arthritis, and Crohn’s disease. By focusing on the immunomodulatory factor IL-23, they found that its proinflammatory activity is strongly dependent on its receptor IL-23R for its structural activation. The research was published in Immunity recently.
Psoriasis, rheumatoid arthritis, inflammatory bowel disease and multiple sclerosis have become more prevalent over the past decades, with an estimated 125 million people affected by psoriasis worldwide and another 100 million affected by rheumatoid arthritis. In addition, although inflammatory bowel disease is associated with ethnicity, the incidence of past uninfested areas has also skyrocketed in recent years. The cytokine IL-23, a special immunomodulatory protein, plays an important role in these diseases. Therefore, IL-23 has become the focus of treatment of these diseases.
Highlights
- Determined crystal structure of human IL-23 in complex with cognate IL-23R
- IL-23 is restrained upon IL-23R binding to enable recruitment of IL-12Rb1
- A single residue in IL-23 is crucial for its pro-inflammatory activity
- Receptor binding sites on IL-23 segregate to the p19 and p40 subunits
Role reversal: When receptors activate cytokines
Interleukin-23, an IL-12 family cytokine, plays pivotal roles in pro-inflammatory T helper 17 cell responses linked to autoimmune and inflammatory diseases. Although IL-23 was discovered 15 years ago, the structural and molecular basis of its pro-inflammatory activity has never been known. Savvids and colleagues now reveal at least one way that IL-23 interacts with one of its receptors. In general, cytokines activate receptors. Surprisingly, however, the current study shows that the opposite occurred.
Professor Savvids said: “We were also surprised when we found that both IL-23 and its receptors changed drastically to create an intimate cytokine-receptor interface on which IL-23 recruits a co-receptor that is essential for pro-inflammatory signaling, and the co-receptor’s binding site on IL-23 is also an unexpected finding. We now report the IL-23-mediated inflammation former complex seems to be a new discovery in this area.”
Continue to combine professional knowledge
The researchers relied on a comprehensive knowledge of structural biology to complete the study. They combined methods of describing protein structure at the atomic level with methods of biochemistry, biophysics, cells, and in vivo studies.
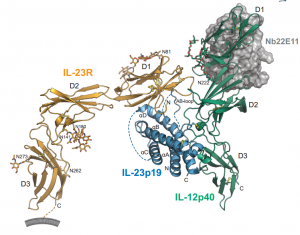
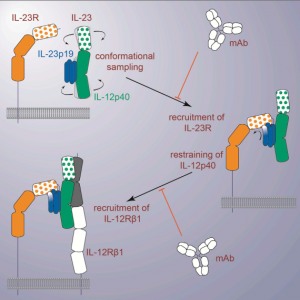
The author determined a crystal structure of human IL-23 in complex with its cognate receptor, IL-23R, and revealed that IL-23R bound to IL-23 exclusively via its N-terminal immunoglobulin domain. The structural and functional hotspot of this interaction partially restructured the helical IL-23p19 subunit of IL-23 and restrained its IL-12p40 subunit to cooperatively bind the shared receptor IL-12Rb1 with high affinity. Together with structural insights from the interaction of IL-23 with the inhibitory antibody briakinumab and by leveraging additional IL-23: antibody complexes, we propose a mechanistic paradigm for IL-23 and IL-12 whereby cognate receptor binding to the helical cytokine subunits primes recruitment of the shared receptors via the IL-12p40 subunit.
Professor Savvids said: “Our initial study on IL-23 will be the cornerstone of our laboratory and other researchers. After all, there are many issues that need to be addressed, such as how IL-23 binds to other possible co-receptors? Our research will likely facilitate the development of new therapeutic strategies for IL-23.”
Reference
loch et al. Structural Activation of Pro-inflammatory Human Cytokine IL-23 by Cognate IL-23 Receptor Enables Recruitment of the Shared Receptor IL-12Rβ1, Immunity (2017). DOI: 10.1016/j.immuni.2017.12.008