Background of Amyloid Proteins Aggregation
The amyloid β (Aβ) monomer that is present in neurons in the human brain performs important tasks for neurons. However, in the brains of patients with Alzheimer’s Disease (AD), the Aβ monomer (mainly 40 and 42 amino acid residues in length) has lost its normal physiological function and spontaneously self-assembles to form aggregates and then protofibrils, which are a heterogeneous class of soluble prefibrillar species with a characteristic secondary and super-secondary structure. The protofibrils continue to form fibrils, which can finally form large deposits called plaques. It is now widely agreed that the soluble Aβ oligomers, rather than amyloid plaques, constitute the primary cause of toxicity, and Aβ42 seems to be the most toxic Aβ isoform owing to its high tendency to form fibrillary and non-fibrillary aggregates.
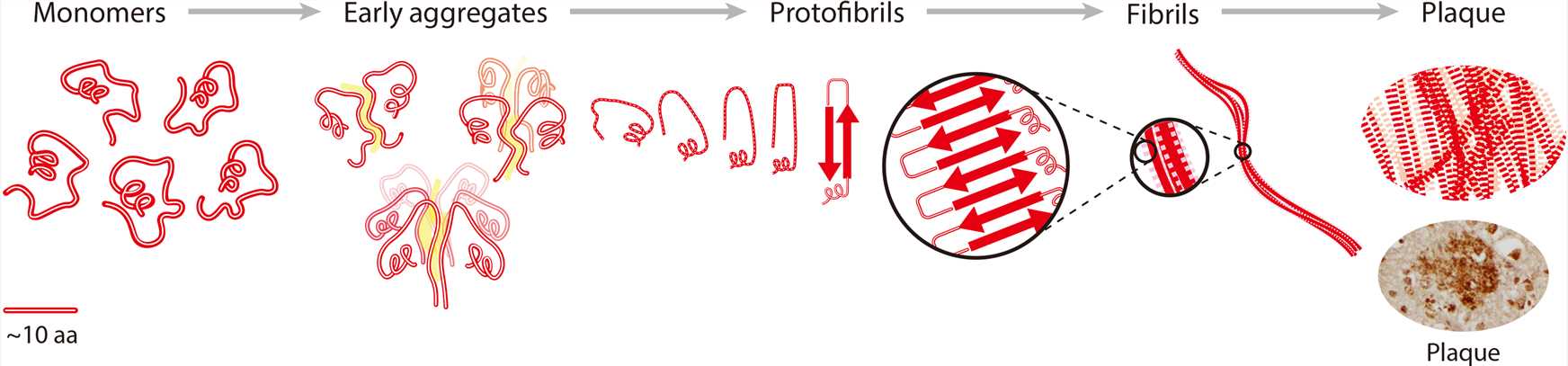
Figure 1. Schematic diagram of Aβ aggregation process from monomer to plaque. (Graham W V.; et al. Update on Alzheimer’s disease therapy and prevention strategies. Annual Review of Medicine. 2017, 68(1): 413-430.)
The Aβ aggregation process is extremely complicated, and consists of at least four microscopic chemical events:
| Microscopic Chemical Event | Description |
| Primary Nucleation of Monomers | Monomers interact with each other in solution to form initial small soluble aggregates. |
| Elongation of Fibrils | Existing fibrils increase in length by the addition of monomers. |
| Fibril Fragmentation | Existing fibrils break apart, increasing the total number of fibrils. |
| Secondary Nucleation of Monomers on Fibril Surface | The surface of existing aggregates catalyzes the formation of new small soluble aggregates. It is considered that most toxic species are generated from the process. |
The non-linearity of this process produces a heterogeneous population of amyloid protein species, which means that pathological Aβ aggregation can be prevented by targeting specific molecular intermediates in the pathway from monomers to plaques or specifically targeting steps involved in toxicity.
Strategies and Challenges in Inhibiting Amyloid Proteins Aggregation
Various strategies have been utilized to interfere with the aggregation process by targeting diverse amyloid conformation species:
Many of these strategies show promising inhibition of toxic amyloid aggregation, but so far none has led to approved therapeutics due to unresolved issues such as blood-brain barrier (BBB) penetration, side effects, target selectivity, and so on.
Development Priorities
Alzheimacy is concerned with the elucidation of the mechanisms of toxicity of amyloid oligomers and the identification of microscopic chemical events during amyloid proteins aggregation to derive specific targeted inhibitors. In addition to small molecular compounds and engineering antibodies, Alzheimacy supports some novel strategies.
If you are interested in this and looking forward to technical support, please feel free to contact us for more details.