The regulation of tau gene expression and splicing mechanisms are emerging targets based on tau pathophysiology. As pathological tau protein is not only directly toxic to cells, but also may be a mediator of amyloid β(Aβ) toxicity, reducing tau levels may be a logical therapeutic approach for Alzheimer’s disease (AD).
Human tau protein is encoded by the microtubule-associated protein tau (MAPT) gene, which contains 16 exons on chromosome 17q21. The adult brain contains six major tau isoforms generated by alternative splicing of exon 2, exon 3 and exon 10. Tau isoforms have different cellular and laminar distribution in the human brain. AD is classified as a 3R+4R tauopathies (3R or 4R refers to the presence of three or four carboxy-terminal repeat domains) according to the tau isoforms found in the aggregates.
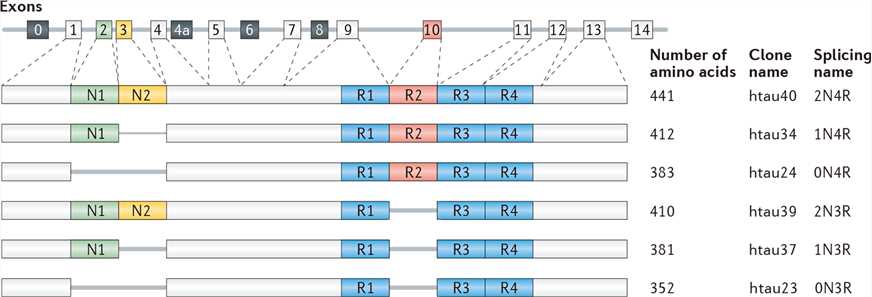
Figure 1. The MAPT gene and tau isoforms in the human brain. (Wang Y, Mandelkow E. Tau in physiology and pathology. Nature Reviews Neuroscience. 2016, 17(1): 22.)
Tau was initially identified as a microtubule-associated protein (MAP). In addition to regulating microtubule dynamics, tau also plays an important physiological role in regulating axonal transport. Structurally, tau belongs to the group of natively disordered proteins and is of high hydrophilicity and little tendency to aggregate. However, tau aggregation is one of the main pathological features of AD.
At present, the trigger factors of tau pathology are still unclear. In familial tauopathies, the mutation of MAPT may be the root cause of tau pathology, but in sporadic tauopathies like AD, tau aggregation, loss of physiological function, toxic gain of function and mislocalization have each been implicated in tau-mediated neurodegeneration. Although tau entities or events that trigger neurotoxicity in AD remain controversial, the reduction in tau expression may have therapeutic implications.
Strategies and Challenges in Targeting Tau Expression
Strategies for direct targeting of MAPT gene expression using antisense oligonucleotides (ASOs), microRNAs (miRNAs), small interfering RNAs (siRNAs) or other transcription inhibitors are promising, and several preclinical studies of antisense therapy have demonstrated possible therapeutic benefits of tau reduction.
Tau-knockout mouse models showed no significant pathology, which may be due to the compensation mechanism of other MAPs, however, deficits in tau could have adverse consequences given the importance of tau in multiple aspects of physiological function of the nervous system. Therefore, it is important to determine the tolerability of diverse levels of tau-knockdown, which can help us minimize any undesirable effects.
Alzheimacy has comprehensive basic research and preclinical drug development platforms that allow us to accelerate your research on tau pathophysiology and development of tau-related therapeutics for AD. If you are interested in this, please feel free to contact us.