Early potential anti-tau therapeutics were based primarily on inhibiting pathological tau phosphorylation or aggregation, or on stabilizing microtubules, but most of these approaches have been discontinued due to adverse reactions or lack of efficacy. Currently, most of the tau-targeting therapeutics in clinical trials are immunotherapies, among which two tau vaccines and eight humanized tau antibodies have entered the clinical trials for Alzheimer’s disease (AD).
Potential Modes of Action of Anti-tau Antibodies
In AD, tau protein exists as monomers, small oligomers, paired helical filaments (PHFs) and straight filaments, and the tau aggregation may be affected by many aberrant post-translational modifications and truncations. Moreover, pathological tau species can be actively transferred between neurons, and even spread tau pathology throughout the brain.
Therefore, therapeutic antibodies or active vaccines are designed to target a variety of tau species in the intracellular or extracellular space. Theoretically, anti-tau antibodies, together with microglia-mediated phagocytosis and protein degradation systems, target and clear either monomeric, aggregated forms, phospho-specific, or conformationally altered forms of tau protein, thus reducing tau aggregation and transmission of pathological tau seeds.
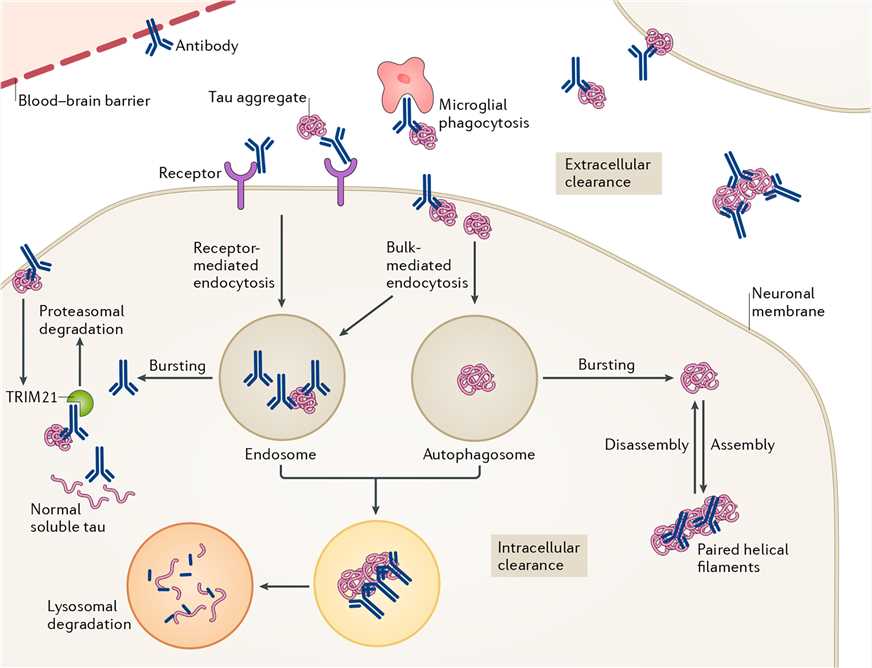
Figure 1. Potential modes of action of anti-tau antibodies. (Congdon E E, Sigurdsson E M. Tau-targeting therapies for Alzheimer disease. Nature Reviews Neurology. 2018, 14(7): 399.)
Anti-tau antibodies also differ in their binding site on tau. Some recognize the N-terminus or the C-terminus, while others show the ability to bind to the proline-rich region or the microtubule binding region (MTBR). Because the conformational changes in the N-terminus of tau occur early in the pathogenesis of AD, it has become an attraction for the preclinical development of tau therapeutic antibodies. The C-terminal fragments are more likely to form filaments than the N-terminal sequences, so antibodies against this binding site are also being studied in preclinical researches. Antibodies recognizing the central region of tau aim to prevent seeding and spreading of pathological tau. The last class of antibodies targets the MTBR, which is responsible for the pathological tau-tau interaction under pathological conditions.
Challenges of Tau Immunotherapies
Active immunotherapy and passive immunotherapy have their advantages and disadvantages. Active immunization is long-lasting, producing polyclonal antibodies that recognize multiple epitopes on the target protein with different affinity and avidity, and is cost-effective to produce, however, there are risks of adverse immune reactions and harmful targeting of the normal protein. Passive immunization requires no immune response from the immune system, and antibodies are precisely characterized in vitro and in vivo to provide greater specificity, but antibodies have a short half-life and are required chronic systemic administration.
In addition, various factors control the efficacy of anti-tau antibodies, the most important of which may be the epitope and the site of action. And the most potent antibodies may be those that target all pathological tau species, both intracellularly and extracellularly.
Alzheimacy has a comprehensive platform for basic research and preclinical research and development, enabling us to accelerate your understanding of tau pathology and the development of tau Immunotherapies for AD. Please let us know if it interests you or if you would like to set up a technical/scientific call with us to explore more details.