γ-secretase (Gamma-secretase, GS) is involved in the cleavage of the amyloid precursor protein (APP) in the amyloidogenic pathway. Specifically, β-secretase cleaves near the N-terminus of the Aβ domain of APP to generate secreted APPβ (sAPPβ) and a membrane-bound C-terminal fragment (C99) containing the entire Aβ domain, which is further cleaved by γ-secretase in a series of steps to generate Aβ peptides of different lengths, of which Aβ40 and Aβ42 are the main products. The efficiency of the successive γ-cleavage affects the production ratio of toxic Aβ42 to total Aβ.
γ-secretase is a multi-subunit enzyme complex with proteolytic activity, consisting of four different membrane proteins, namely, presenilin (PS1), presenilin enhancer 2 (PEN-2), nicastrin (NCT) and anterior pharynx-defective 1 (APH-1). PS1 is the catalytic subunit of γ-secretase. γ-secretase can cleave multiple type I transmembrane proteins (over 90 reported substrates), of which APP and Notch are the best-characterized substrates.
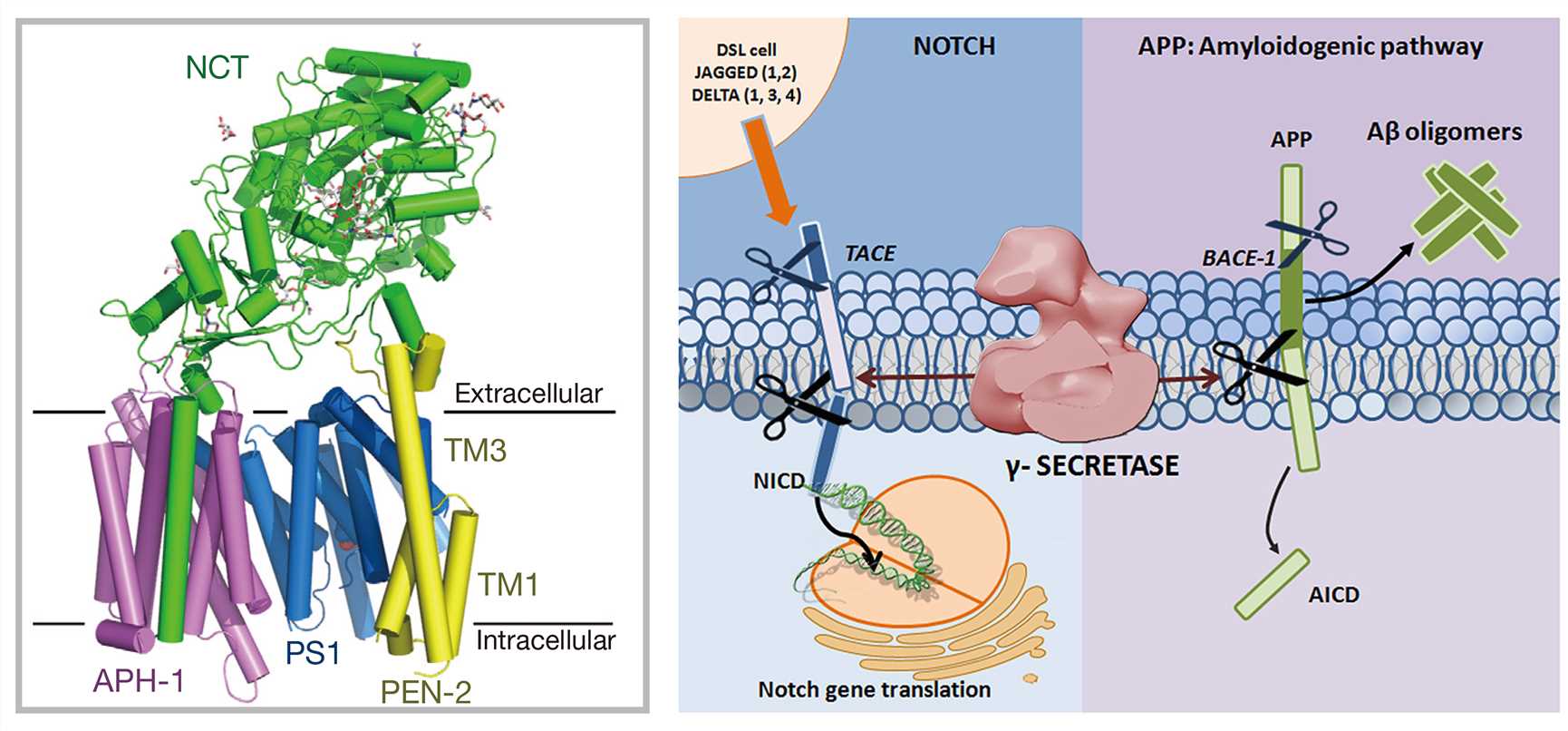
Figure 1. Atomic structure of γ-secretase and its involvement in proteolytic processing of APP and Notch. (Adapted from Bai X.; et al. An atomic structure of human γ-secretase. Nature. 2015. 525: 212-217; Gertsik N.; et al. Complex regulation of gamma-secretase: from obligatory to modulatory subunits. Frontiers in Aging Neuroscience. 2015, 6: 342.)
Current Situation and Challenges of γ-secretase as the Target of AD
It is difficult to modulate Aβ production by regulating γ-secretase, in part because of the complexity of the γ-secretase complex. The regulation of γ-secretase complex activity is still far from being well understood. The four subunits that make up the complex and γ-secretase-associated proteins are potential therapeutic targets.
It is now known that γ-secretase complex cleaves many different type I transmembrane protein substrates besides APP. In particular, γ-secretase plays a crucial role in controlling the proteolysis of the transmembrane domain of the Notch receptors. Inhibition of γ-secretase by a γ-secretase inhibitor (GSI) affects Notch signaling and can lead to severe adverse reactions. Therefore, the identification of highly selective and specific inhibitors poses a great drug-development challenge.
Development Strategy and Focus
Regarding the development of Alzheimer's therapeutics targeting γ-secretase, Alzheimacy hopes to help you in the development of a new generation of GSIs and γ-secretase modulators (GSMs) that can selectively inhibit APP cleavage by γ-secretase or block the APP binding site rather than the active site of γ-secretase.
Alzheimacy is concerned with relatively safer disease-modifying therapies. For example, GSM that can modulate the activity of γ-secretase, reduce the production of toxic Aβ42 by shifting the APP cleavage toward the production of shorter and less aggregating Aβ peptides, without affecting cleavage of Notch or other γ-secretase substrates. We also focus on novel targets, for instance, regulating the γ-secretase complex by targeting γ-secretase-associated proteins. Moreover, we support further develop basic research on γ-secretase biology to advance the design of specific GSIs and GSMs. If you are interested in this and looking forward to technical support, please feel free to contact us.