Background of Amyloid β Production
Amyloid β (Aβ) refers to a group of hydrophobic peptides composed of 39~43 amino acid residues, predominantly Aβ40 and Aβ42, whose pathological aggregation is thought to be implicated in neuronal degeneration and cognitive decline in Alzheimer’s Disease (AD). The Aβ peptides are proteolytic fragments derived from amyloid precursor protein (APP), which is a large type I transmembrane protein, and there are three known pathways to process APP.
| Pathway | Description |
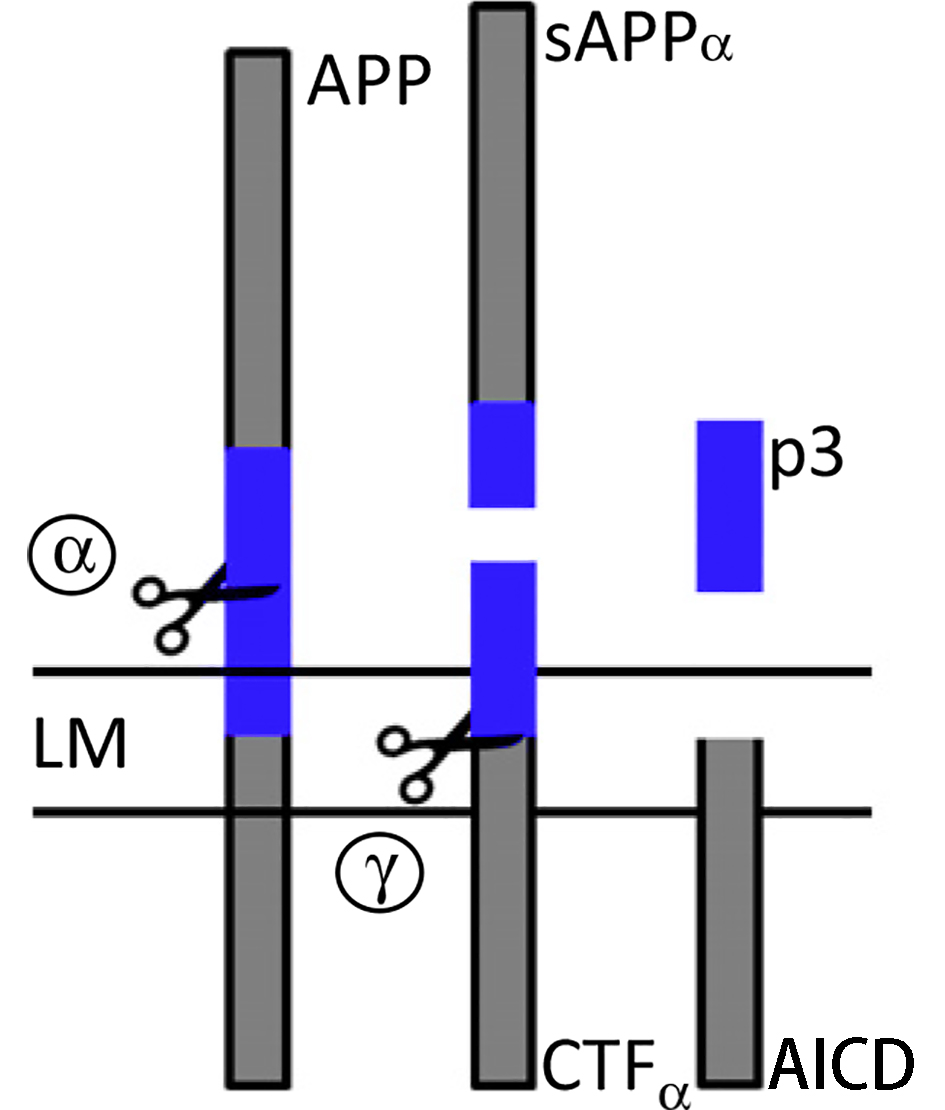 |
The non-amyloidogenic pathway is the primary pathway under physiological conditions. APP is first cleaved by α-secretase, generating the soluble ectodomain sAPPα and leaving the C-terminal fragment α (CTFα) in the plasma membrane. CTFα is subsequently cleaved by γ-secretase to release the soluble extracellular p3 peptide and the APP intracellular domain (AICD). |
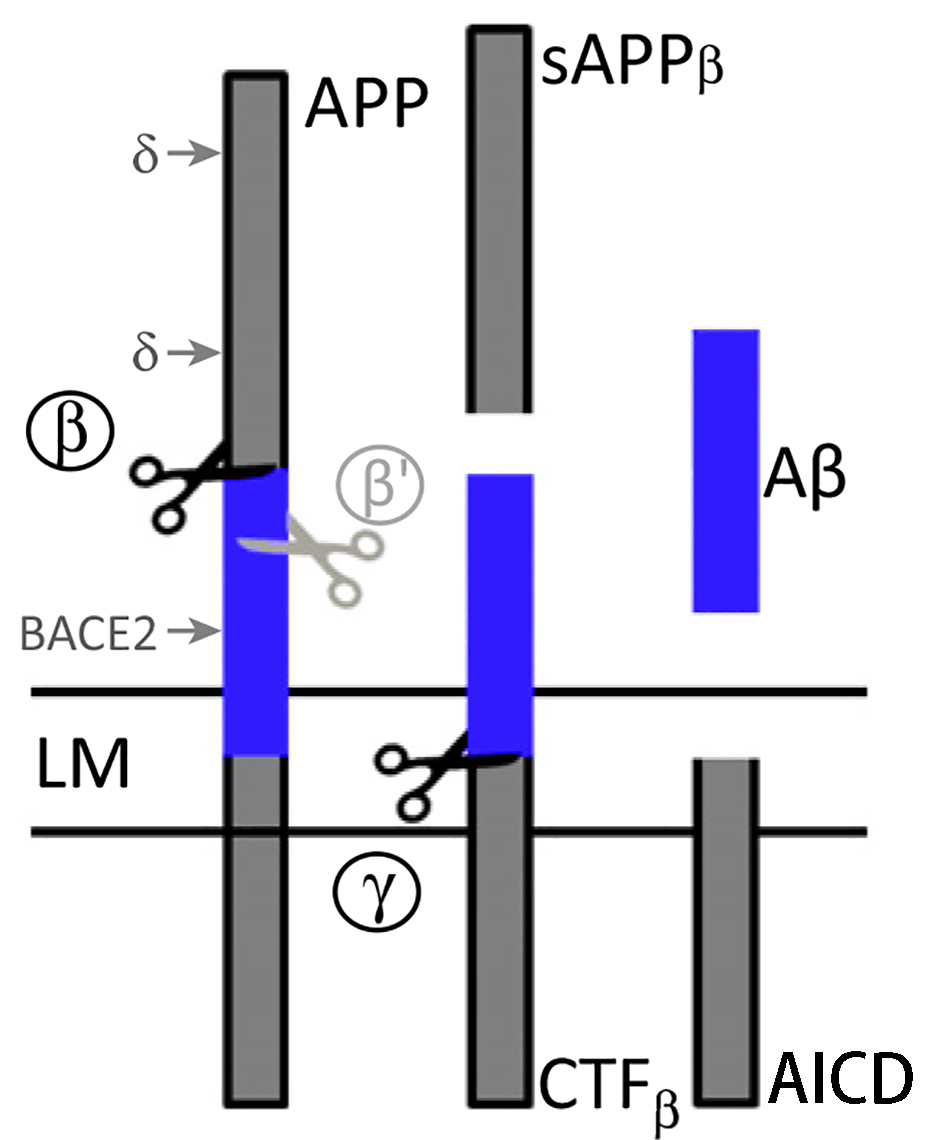 |
The first step of the amyloidogenic pathway is catalyzed by β-secretase, which cleaves APP to generate the soluble ectodomain sAPPβ and the CTFβ left in the membrane. Subsequently, the CTFβ is cleaved by γ-secretase to produce Aβ monomers and AICDs. Moreover, γ-secretase cleaves the CTFβ at different sites and in multiple successive steps, producing various Aβ species (including two major Aβ peptides, namely Aβ40 and Aβ42). |
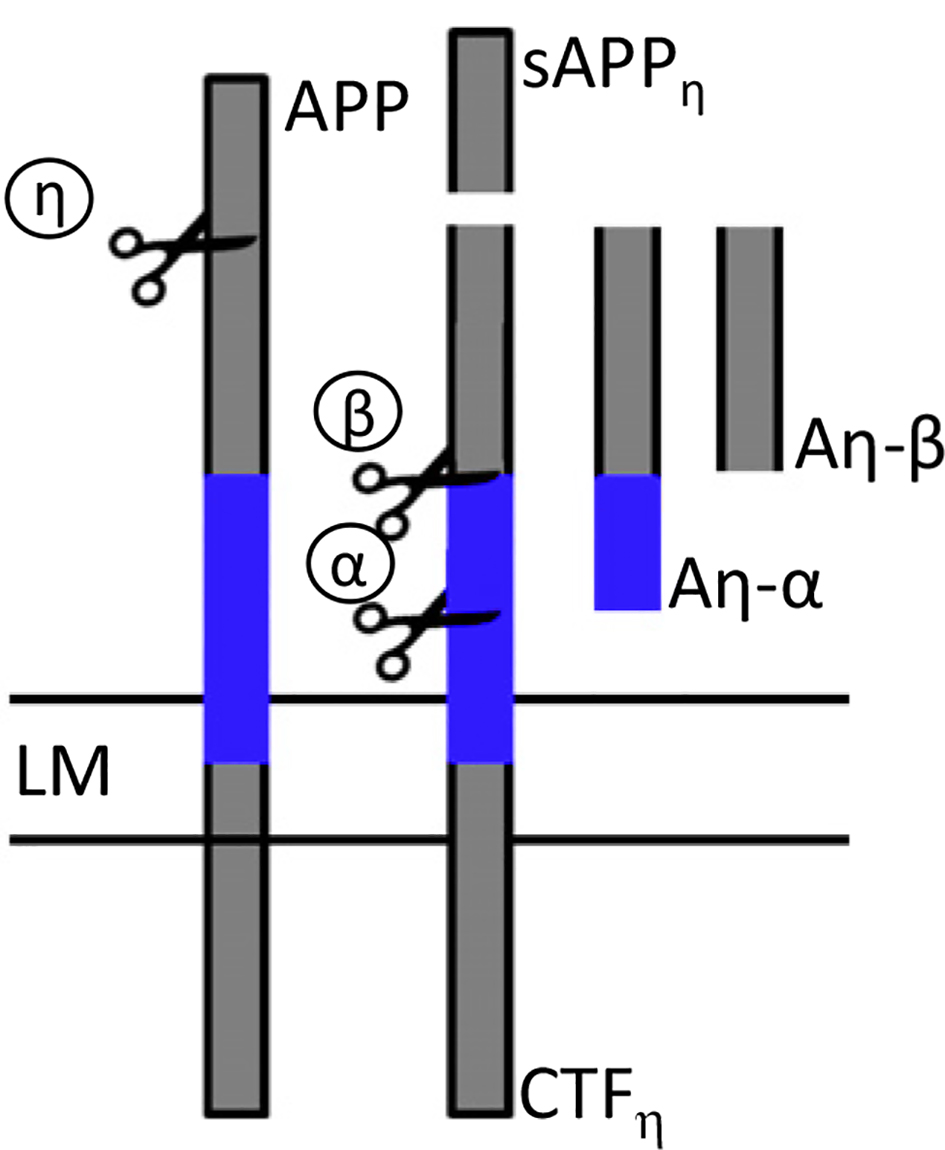 |
A new APP processing pathway has recently been identified. This pathway yields proteolytic fragments derived from APP capable of inhibiting neuronal activity within the hippocampus. The cleavage of APP by η-secretase generates CTFη, which is cleaved by α-secretase and β-secretase into long and short amyloid η (Aη-α and Aη-β). There may be a connection between this pathway and the amyloidogenic pathway. |
Strategies and Challenges in Modulating Aβ Production
The amyloid cascade hypothesis suggests that the pathological aggregation of Aβ is one of the main causes of AD. Thus, reducing Aβ production has become an attractive R&D direction, particularly with a strong interest in β-secretase and γ-secretase as potential targets for drugs that might reduce Aβ production.
Although considerable efforts have been made to develop drugs targeting these targets, numerous clinical trials have failed due to poor brain permeability or severe side effects of candidate agents.
Alzheimacy has not abandoned the strategy of modulating Aβ production to develop AD therapy and hopes to help you put forward some novel ideas based on a more in-depth understanding of the mechanisms involved in amyloid proteins. Please let us know if it interests you or if you would like to set up a technical/scientific call with us to explore more details.