Tau is a natively unfolded protein that is highly soluble and shows little tendency for aggregation. The highly flexible structure of tau allows it to interact with multiple partners, indicating its involvement in many signaling pathways. However, this structure also gives it the ability to interact with other tau molecules to form oligomers and filaments. The conformational changes and oligomer formation of tau represent the early event of tau pathology in Alzheimer’s disease (AD).
Post-translational Modification and Tau Aggregation
Under pathological conditions, tau undergoes various aberrant post-translational modifications (PTMs), such as truncation, phosphorylation, ubiquitination, acetylation, glycosylation, etc. Among them, truncation and phosphorylation are the most investigated. It is considered that PTMs of tau (except O-GlcNAcylation) interfere with tau-microtubule (MT) binding, induce misfolding of tau, and thus contribute to tau aggregation. Therefore, targeting any of these PTMs has the potential to prevent tau aggregation and restore tau physiological functions.
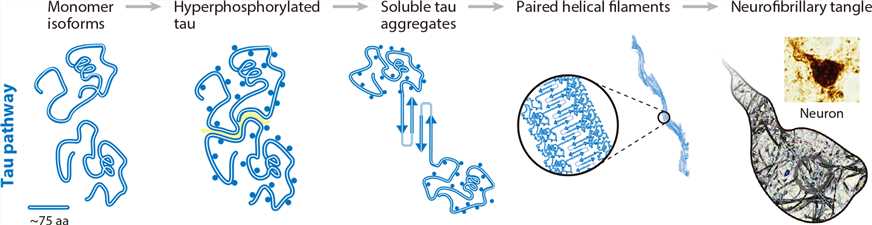
Figure 1. Schematic diagram of tau aggregation process from monomers to NFTs. (Graham W V.; et al. Update on Alzheimer’s disease therapy and prevention strategies. Annual Review of Medicine. 2017, 68(1): 413-430.)
The truncated tau has been shown to be a driving force of neurofibrillary degeneration. And truncated tau fragments containing repeat domains tend to aggregate and lose cytoskeletal MT-stabilizing properties. Hyperphosphorylation of the tau protein (primarily at Ser and Thr residues) affects its ability to bind tubulin and promote MT assembly, resulting in increased levels of unbound tau, thus increasing the possibility of tau-tau interactions and polymerization. Tau phosphorylation is strictly controlled by various protein kinases and phosphatases, among which glycogen synthase kinase 3β (GSK-3β) and phosphatase 2A (PP2A) are two key enzymes involved in regulating tau phosphorylation state. The degree of tau phosphorylation during AD development reflects the abnormal activity of protein kinases and phosphatases. Tau phosphorylation is also regulated by O-GlcNAcylation. Tau undergoes PTMs at Ser/Thr residues by O-linked N-acetylglucosamine (O-GlcNAc), and thus the O-GlcNAcylation of tau protects it from phosphorylation.
| Therapeutic Strategies | |
| Compounds | Kinase inhibitors, Phosphatase activators, O-deglycosylation inhibitors |
| Immunotherapies | Therapeutic antibodies against various epitopes |
Tau Oligomerization
In AD, the brain tissue contains multiple tau multimer species, including paired helical filaments (PHFs), straight filaments, and small oligomeric aggregates. Oligomeric tau has become the candidate for the most neurotoxic tau species. Moreover, soluble tau aggregates may also affect membrane integrity and are involved in the spreading of tau pathology. Thus, preventing or reversing tau oligomerization has the potential to improve cell health and prevent the spread of tau pathology to other brain regions.
| Therapeutic Strategies | |
| Compounds | Direct inhibitors of tau protein aggregation |
| Immunotherapies | Tau-specific antibodies against oligomeric tau |
Protein Degradation Pathway Impairment
Protein degradation pathways including the autophagy pathway and the ubiquitin-proteasome system are dysfunctional in AD, while protein quality control plays a significant role in preventing tau aggregation and reducing levels of neurotoxic tau species. Impaired protein degradation pathways may lead to the accumulation and eventual aggregation of misfolded tau protein.
| Therapeutic Strategies | |
| Compounds | Modulators of autophagy or proteasomal degradation |
In addition, continuing basic research on how tau aggregation exerts neurotoxicity is critical to identify novel targets for intervention. Alzheimacy has a comprehensive basic research and preclinical drug development platform, and if you are interested in tau pathology research and drug development targeting tau aggregation, please feel free to contact us.