Background
It is well known that the production of neurotoxic amyloid beta (Aβ) is dependent on the processing of amyloid precursor protein (APP), which is a key protein involved in the pathology of Alzheimer’s Disease (AD). However, APP also has non-pathogenic functions, not only providing neuroprotection after processing through the non-amyloidogenic pathway, but also playing an indispensable role in regulating intracellular iron homeostasis.
Key factors leading to the pathogenicity of Aβ peptides through dysregulated processing of APP include the switch from the non-amyloidogenic pathway to the amyloidogenic pathway, in which APP is cleaved by β-secretase and γ-secretase to produce the APP intracellular domain (AICD) and amyloidogenic Aβ40 and Aβ42. In the non-amyloidogenic pathway, APP is cleaved by α-secretase and γ-secretase to generate the AICD and non-amyloidogenic P3 peptide, both of which are degraded and internalized.
Moreover, the mRNA 5’-untranslated region (5’-UTR) of APP has a stem-loop structure homologous to the iron-responsive element (IRE) of the ferritin and ferroportin (FPN). The IRE can bind to the iron regulatory protein (IRP), which modulates APP mRNA translation and stability based on intracellular iron levels. In addition, APP has ferroxidase activity, assisting FPN in iron export and neuronal iron efflux. In AD, brain perturbation of iron metabolism is observed and may be related to APP overexpression.
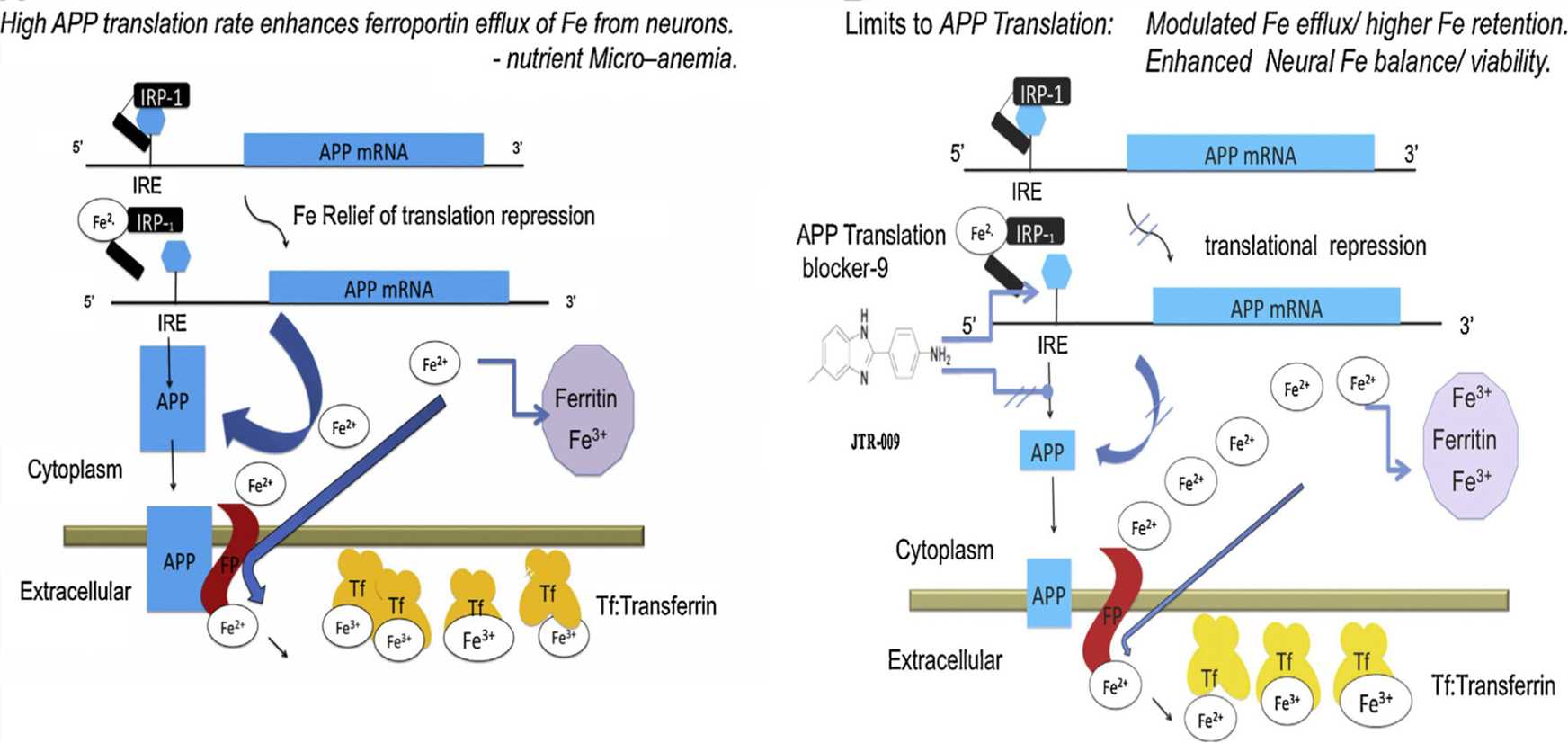 Figure 1. Model for cooperative translation of APP by IRP1 and facilitated FPN dependent export. (Bandyopadhyay S, Rogers J T. Alzheimer’s disease therapeutics targeted to the control of amyloid precursor protein translation: Maintenance of brain iron homeostasis. Biochemical Pharmacology. 2014. 88(4): 486-494.)
Figure 1. Model for cooperative translation of APP by IRP1 and facilitated FPN dependent export. (Bandyopadhyay S, Rogers J T. Alzheimer’s disease therapeutics targeted to the control of amyloid precursor protein translation: Maintenance of brain iron homeostasis. Biochemical Pharmacology. 2014. 88(4): 486-494.)
Therefore, the strategy of regulating APP levels by limiting APP expression can be employed to reduce brain amyloid burden and improve the brain iron homeostasis.
5’-UTR of APP mRNA as the Target of AD
APP mRNA encodes a highly active IRE that binds with a different RNA binding specificity to IRP-1 relative to the ferritin IRE, thus enabling APP mRNA 5’-UTR to provide specificity as a target to identify small molecules to suppress the generation of APP and toxic Aβ peptides.
Alzheimacy supports the strategy to selectively limit the translation of APP mRNA. We will not ignore basic research related to this strategy. In addition to 5’-UTR of APP mRNA, we also support the exploration of whether the 3’-UTR of APP mRNA can control protein levels and whether it can serve as a potential drug target. If you are interested in this and looking forward to technical support, please feel free to contact us.