In a new preclinical study, researchers from the Perelman School of Medicine at the University of Pennsylvania found that as a method of reprogramming patients’ autoimmune cells to attack their blood cancer, CAR-T cell therapy may improve the effectiveness of surgical treatment of solid tumors. The relevant research results were published in the journal Science Advances, and the title of the paper was “Chiric antigen receiver T cells as adjuvant-therapy for unresectable adenoccinoma”.
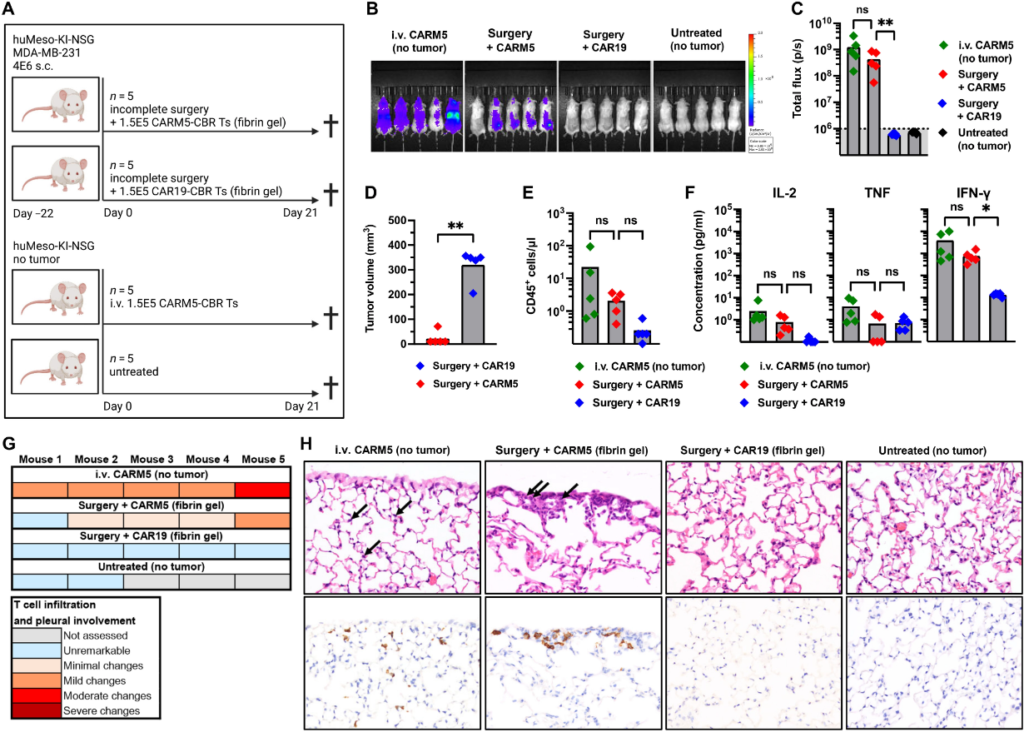
In this new study, the authors applied a special gel containing human CAR-T cells to the surgical wound after partial tumor resection in mice. They found that in almost all cases, CAR-T cells apparently eliminated the residual tumor cells, thus enabling these mice to survive, otherwise, they would die of tumor recurrence.
When the solid tumor does not spread, surgery can be curative. However, for surgeons, it is often difficult to distinguish the endpoint of a tumor from the starting point of healthy tissue. Therefore, for many types of cancer, postoperative recurrence caused by residual small tumor cells is very common. One possible way to solve this problem is to carry out anti-tumor treatment on the edge of the remaining tissue immediately after tumor resection to kill any remaining tumor cells. In this new study, these authors tested this method using CAR-T cells.
Dr. Carl June, the corresponding author of the paper and director of the Cellular Immunotherapy Center of Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, said, “As we continue to promote CAR-T cell therapy, it is a major goal to use it to treat solid tumors. Based on the gratifying results of this new study, our colleagues have planned to conduct clinical trials in patients with locally advanced breast cancer.”
CAR-T cells are powerful immune cells – T cells – genetically modified to express chimeric antigen receptors (CAR) targeting specific proteins. All CAR-T cell therapies approved for clinical use target proteins on the surface of cancer cells. Normally, T cells are obtained from the blood of patients, genetically modified in the laboratory, and then transplanted back into the patient to play a role as “living drugs”.
June and her colleagues helped develop and test the first CAR-T cell therapy approved by the US Food and Drug Administration (FDA) in 2017. Now there are six approved CAR-T cell therapies for the treatment of multiple blood cancers, which provides new hope for patients who have no conventional treatment options.
So far, the solid tumor has been a more difficult target for CAR-T treatment, partly due to tumor volume and tumor anti-immune defense. However, another group of researchers found in a study last year that in a mouse model of brain cancer, CAR-T cells may be useful for the arduous task of removing residual cancer cells after surgery.
In this new study, June and her colleagues tried the same method for two other types of cancer – triple-negative breast cancer and human pancreatic duct cancer. Among them, triple-negative breast cancer lacks all three major breast cancer markers, and human pancreatic duct cancer is the most common type of pancreatic cancer. Both solid tumors are extremely difficult to cure.
In these experiments, they designed CAR-T cells to target a protein called mesothelin, which is the surface marker of these two types of tumor cells. If the fibrin gel they designed containing CAR-T cells is not given, the remaining tumor tissue will grow and the mice will die in about seven weeks. However, after the delivery of this fibrin gel containing CAR-T cells, the residual tumor tissue in 19 of the 20 mice disappeared rapidly, and these 19 mice had no wound healing complications or other obvious side effects in the rest of the observation period.
Further experiments show that CAR-T cells targeting mesothelin may attack healthy cells carrying this protein marker after intravenous injection, and the toxicity of local injection of these CAR-T cells is lower than that of direct injection of these CAR-T cells into the blood.
June said, “This new study shows the prospect of CAR T as adjuvant therapy after solid tumor surgery. We also believe that in addition to CAR-T cells, this method may also be extended to deliver other cell therapies and anticancer drugs, which has the potential to further improve the anti-tumor effect.”
Reference
Ugur Uslu et al. Chimeric antigen receptor T cells as adjuvant therapy for unresectable adenocarcinoma. Science Advances, 2023, doi:10.1126/sciadv.ade2526.
CAR T Cell Therapy May Eliminate Tumor Cells Missed by Surgery