An international study lasting for 13 years has shown for the first time that the degradation of the way DNA is assembled and regulated – the so-called epigenetics – can drive the aging of organisms, and has nothing to do with the change of the genetic code itself. This study shows that the destruction of epigenetic information leads to the aging of mice, and the restoration of the integrity of the epigenome can reverse these signs of aging. The relevant research results were published online in Cell and the title of the paper was “Loss of epigenetic information as a cause of mammalian aging”.
David Sinclair, the co-author of the paper and professor of genetics at the Brawanik Institute of Harvard Medical School, said, “We believe that our study is the first to show that epigenetic changes are the main driving factor of mammalian aging.”
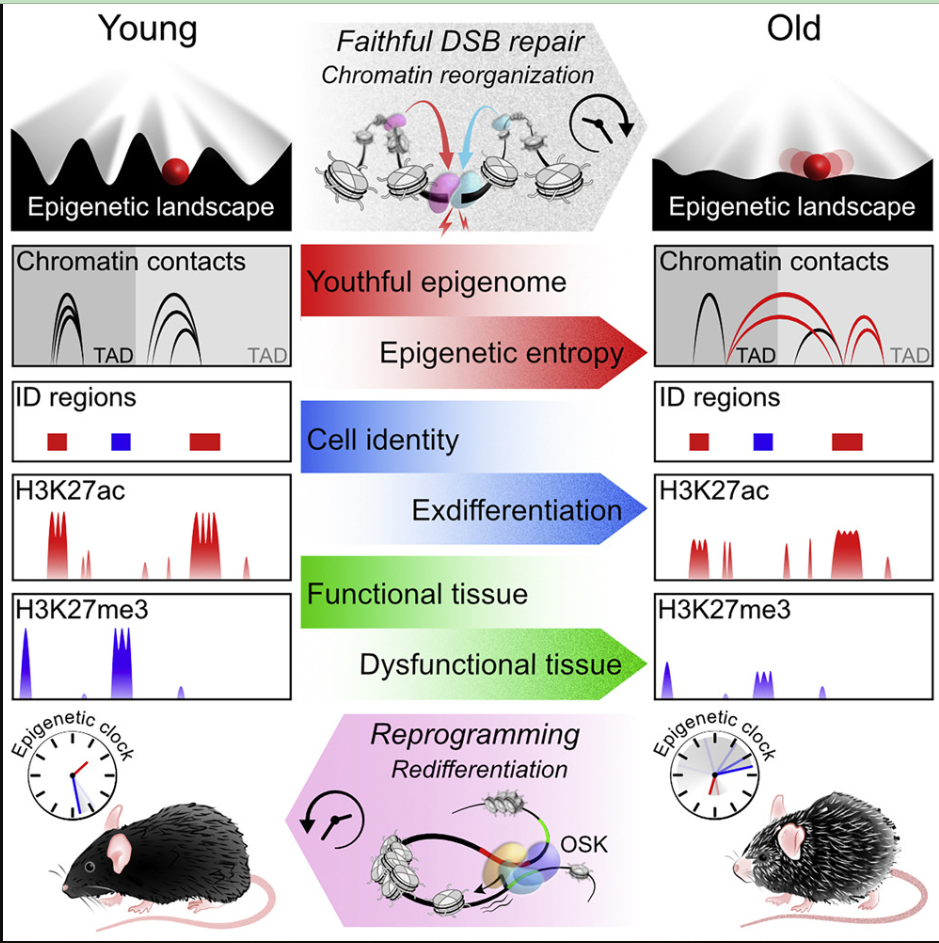
A series of extensive experiments by these authors provide long-awaited confirmation that DNA changes are not the only or even the main cause of aging. On the contrary, their research results show that the chemical and structural changes of chromatin – the complex of DNA and protein that forms chromosomes – accelerate aging without changing the genetic code itself.
Jae-Hyun Yang, co-author and co-first author of the paper and genetic researcher of Sinclair Laboratory, said, “We expect that these findings will change the way we view the aging process and the way we treat diseases related to aging.”
These authors said that since it is easier to manipulate molecules that control epigenetic processes than to reverse DNA mutations, this new study points out a new way to focus on epigenetics rather than genetics to prevent or treat age-related damage.
First, these results need to be verified in large mammals and humans. Research on non-human primates is currently underway. Sinclair said, “We hope these results can be seen as a turning point in our ability to control aging. This is the first study that shows that we can accurately control the biological age of complex animals; we can drive it forward and backward at will.”
Beyond mutation
For those who study aging, the most urgent question may be what causes aging. For decades, a mainstream theory in this field is that aging comes from the accumulation of DNA changes, mainly gene mutations. Over time, more and more genes cannot work properly. These failures, in turn, cause cells to lose their identity, collapse tissues and organs, and lead to disease and eventually death.
However, in recent years, more and more studies have shown that this is not the case. For example, some scientists found that some people and mice with high mutation rates did not show signs of premature aging. Others have observed that many types of aging cells have little or no mutations.
Scientists began to wonder what else could cause aging with or instead of DNA changes. There is a growing list of possible culprits. These include epigenetic changes.
An integral part of epigenetics is the physical structure, such as histones that wrap DNA into compact chromatin and unpack part of DNA when necessary. When genes are wrapped, they are inaccessible, but when they are unwrapped, they can be transcribed and used to produce proteins. Therefore, epigenetic factors regulate which genes are active or inactive in any given cell at any given time.
By acting as a switch of gene activity, these epigenetic molecules help to determine cell type and function. Because every cell in the organism has basically the same DNA, it is the on-off switch of a specific gene that distinguishes nerve cells from muscle cells and lung cells. Yang said, “Epigenetics is like a cell’s operating system, telling it how to use the same genetic material in different ways.”
At the end of the 1990s and the beginning of the 21st century, Sinclair Laboratory and others found that epigenetic changes accompany aging in yeast and mammals. However, they cannot judge whether these changes promote aging or the result of aging. Until this new study, Sinclair and his research team were able to separate epigenetics from genetic changes, and confirmed that the destruction of epigenetic information actually promoted mouse aging.
ICE mice
The main experiment of Sinclair’s team involves the production of temporary, fast-healing DNA breaks (i.e. incisions) on the DNA of laboratory mice. These breaks simulate the low-level and continuous chromosome breaks experienced by mammalian cells every day due to respiration, exposure to sunlight and cosmic rays, and exposure to certain chemicals.
In this new study, to test whether aging comes from this process, Sinclair and his colleagues increased the number of DNA breaks to simulate fast-forward life. They also made sure that most of the breaks were not in the coding region of mouse DNA – the fragments of genes. This prevents mutations in the genes of these mice. Instead, these breaks change the way DNA folds. They call their system ICE, which is short for inducible changes to the epigenome.
At first, epigenetic factors suspended their normal task of regulating genes, and moved to DNA breaks to coordinate the repair. After that, these factors returned to their original positions. But things have changed over time. They noticed that these factors were “distracted” and did not return to the original position after repairing the fracture. The epigenome became disorganized and began to lose its original information. Chromatin condenses and unfolds in the wrong mode, which is a sign of epigenetic dysfunction.
As these mice lose their young epigenetic function, they begin to look and behave very old. These authors observed an increase in biomarkers indicating aging. Cells lose their identity. The organizational function has weakened and organ failure.
These authors use a tool recently developed by Sinclair Laboratory to measure the age of mice, not in chronological order (in days or months), but in accordance with how many sites in the genome have lost the methyl group usually attached to them, to measure “biologically”. Compared with untreated mice born at the same time, the age of ICE mice increased significantly.
Young again
Next, the authors provided these mice with gene therapy to reverse the epigenetic changes they caused. “It’s like restarting a broken computer,” Sinclair said
This gene therapy provides three genes – Oct4, Sox2, and KLF4, collectively named OSK – which are active in stem cells and can help mature cells return to their early state. (Sinclair laboratory used this cocktail to restore the vision of blind mice in 2020). The result is that this method can restore the organs and tissues of ICE mice to a young state. Sinclair said that the treatment “started an epigenetic program, causing cells to restore their epigenetic information when they were young. This is a permanent reset”. It is not clear how it achieved this.
Sinclair said that at this stage, this discovery supports the hypothesis that mammalian cells maintain a backup of epigenetic programs. When activated, aging and epigenetic-disordered cells can be restarted to a young and healthy state. At present, a series of extensive experiments led Sinclair’s team to conclude that “aging can be driven forward and backward by manipulating the epigenome”.
From here on
This ICE method provides a new way for people to explore the role of epigenetics in aging and other biological processes. Since ICE mice show signs of aging only after 6 months rather than at the end of the average life span of mice of two and a half years, this method also saves time and money for scientists studying aging.
In addition to OSK gene therapy, people can also explore how to recover lost epigenetic information in aging organisms. Yang said, “There are other methods to manipulate the epigenome, such as drugs and small molecular chemicals that induce mild stress. This study opens a door for the application of these other methods to restore cells and tissues to youth.”
Sinclair hopes that this new research can inspire other scientists to study how to control aging, so as to prevent and eliminate human age-related diseases and symptoms, such as cardiovascular disease, type 2 diabetes, neurodegeneration, and weakness. He said, “These are all signs of aging. We have been trying to treat them with drugs when they appear, which is almost too late.”
The goal of Sinclair’s team is to solve the root causes of aging, so as to extend the health span of human beings. However, medical applications are still far away, and extensive experiments need to be carried out in a variety of cells and animal models. However, Sinclair said that in order to realize such a dream, scientists should think further and keep trying.
He said, “We are talking about rejuvenating the whole body or a specific organ of the elderly or patients, so that the disease will disappear. This is a great idea.”
Related Services
Reference
Jae-Hyun Yang et al. Loss of epigenetic information as a cause of mammalian aging. Cell, 2023, doi:10.1016/j.cell.2022.12.027.