In a new study, researchers from the University of Health and Science of Oregon in the United States revealed the molecular structure of a receptor crucial for brain development and function – the Type A GABA receptor. The relevant research results were published online in Nature titled “Cryo-EM structures reveal native GABAA receptor assemblies and pharmacology”.
The A-type GABA receptor has become a target for drug anesthetics, sedatives, and antidepressants due to its important role in brain function. These authors have revealed the main combinations and states of this GABA receptor, which helps to develop new compounds that are more targeted towards a range of medical diseases.
Dr. Chang Sun, the first author of the paper and postdoctoral researcher at the Wallam Institute at the University of Health and Sciences in Oregon, said, “It is the main role in balancing brain excitation and inhibition. It affects all aspects of brain function, from motor function to memory and learning, as well as emotions and anxiety.”
Dr. Eric Gouaux, the corresponding author of the paper and senior scientist at the Wallam Institute at the University of Health and Sciences in Oregon, added, “Due to the importance of this switch, type A GABA receptors are distributed throughout the entire brain.”
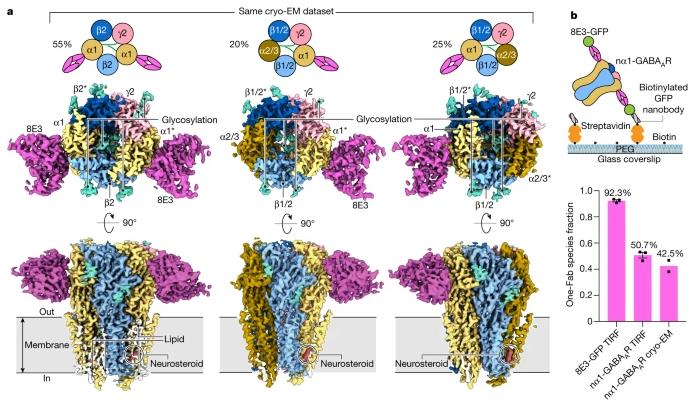
Type A GABA receptors are assembled from pentamers composed of 19 different subunits, each producing a large number of configurations that may or may not be clinically relevant. In this case, these authors painstakingly isolated natural A-type GABA receptor assemblies from mice and provided them with commonly used drugs for treating insomnia and postpartum depression. They were then able to observe the three major structural types of type A GABA receptors in their natural state.
Gouaux said, “This new study shows the main combinations and states of A-type GABA receptors in their natural state. This is the truly significant breakthrough – no one has been able to figure out which assembly has the highest density among the thousands of these A-type GABA receptor assemblies before.”
This discovery demonstrates the natural state of type A GABA receptors, rather than the state during tissue culture. These authors utilized state-of-the-art cryo-EM to reveal the structure of A-type GABA receptors in their natural state.
She said, “We used a combination of cryo-EM and single-molecule microscopy techniques, which allowed us to count the subunits in each A-type GABA receptor pentamer complex.”
Related Services
Protein-Lipids/Nucleic Acid Interaction Assay and Screening
Protein Expression and Purification Services
Reference
Chang Sun et al. Cryo-EM structures reveal native GABAA receptor assemblies and pharmacology: Nature, 2023, doi:10.1038/s41586-023-06556-w.