RAS participates in multiple signaling pathways, and post-translational modifications regulate the subcellular localization of RAS to regulate downstream signal transduction. Among them, palmitoylation is a necessary modification of HRAS and NRAS signal transduction. The acyltransferase DHHC9 binds to the helper protein GCP16 to catalyze palmitoylation of HRAS and NRAS, but the interaction between GCP16 and DHHC9, as well as the molecular mechanism by which GCP16 regulates the palmitoylation activity of DHHC9, remain unclear.
The Hu Qi and Wu Jianping team from the School of Life Sciences at West Lake University collaborated to publish a research paper titled “Regulation of RAS palmitoyltransferases by accessory proteins and palmitoylation” in the journal Nature Structural & Molecular Biology.
This study reported the cryo-electron microscopy structure of the human acyltransferase DHHC9-GCP16 and yeast homologous protein Erf2-Erf4 complex, which catalyzes the palmitoylation of RAS. The regulatory role of auxiliary proteins on acyltransferases was elucidated, and it was found that the binding of phospholipid molecules in DHHC9 and the palmitoylation of three cysteine sites (Cys24, 25, and 288) on non-active centers are crucial for enzyme catalytic activity, And based on the structure, acyltransferases DHHC14 and DHHC18 were discovered that can bind to the helper protein GCP16 and catalyze RAS palmitoylation in addition to DHHC9.
Our Featured Products
| Cat. No. | Product Name | Source (Host) | Species | Tag |
| HRAS-2524H | Recombinant Human HRAS protein(Met1-Cys186), His-tagged | Insect Cells | Human | C-His |
| HRAS-13934H | Recombinant Human HRAS, His-tagged | E.coli | Human | His |
| NRAS-447H | Recombinant Human NRAS Protein, His-tagged | E.coli | Human | His |
| NRAS-01H | Active Recombinant Human NRAS Protein, His-tagged | Bacterial expression system | Human | His |
| GOLGA7-13386H | Recombinant Human GOLGA7, GST-tagged | E.coli | Human | GST |
| ZDHHC9-426H | Recombinant Human ZDHHC9 | Mammalian Cell | Human | His |
The activity of the RAS family is regulated by GTP and GDP, and is inactive when combined with GDP. It is activated when combined with GTP. In addition, RAS activity is also regulated by lipid modification. The C-terminus of RAS has a conserved CAAX (cysteine aliphatic amino acid arbitrary amino acid) motif, and the isopentenylation of the cysteine site transports RAS to the endoplasmic reticulum. The RAS family members HRAS and NRAS also have a second type of lipid modification – palmitoylation, which mediates the transport of HRAS and NRAS to the cell membrane and activates downstream signal transduction.
DHHC9 is a known acyltransferase of HRAS and NRAS. As one of the 23 cysteine acyltransferases in the human body, DHHC9 catalyzes long-chain acyl (usually palmitoyl) modification of the cysteine side chain before the CAAX sequence of RAS. The catalytic activity of DHHC9 depends on the auxiliary protein GCP16, but the interaction between GCP16 and DHHC9 and the regulatory mechanism of DHHC9 enzyme activity are still unclear.
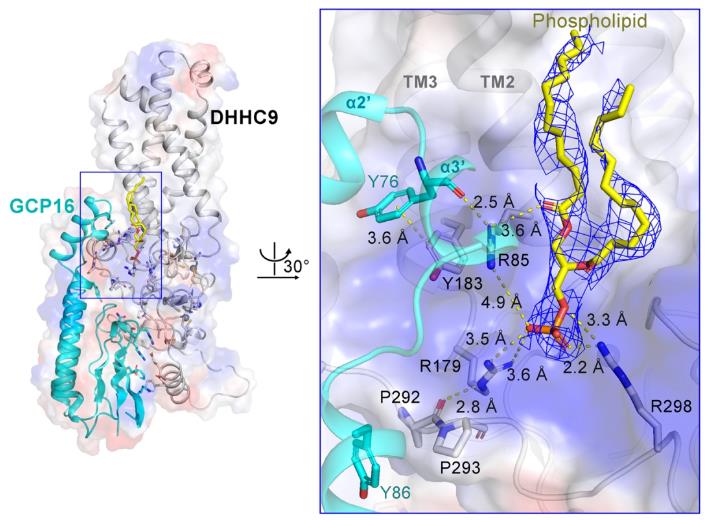
To investigate the regulatory mechanism of auxiliary protein GCP16 on the activity of DHHC9, the team expressed and purified the human DHHC9-GCP16 complex and its homologous protein Erf2-Erf4 complex in yeast, and analyzed their cryo-electron microscopy structures. The structure shows that GCP16 regulates the activity of DHHC9 by stabilizing its structure, rather than directly participating in the catalytic process of DHHC9.
The research team found that there is a phospholipid molecule at the interface between DHHC9 and GCP16, which can stabilize the conformation of DHHC9 and its interaction with GCP16. In addition, the team identified palmitoylation modifications on three cysteine residues (C24, C25, and C288) outside the DHHC9 catalytic center. Biochemical experiments have shown that the modification of these three sites is crucial for the catalytic activity of DHHC9, and site mutations significantly reduce the palmitoylation modification level of DHHC9 on substrates.
Structural analysis and sequence alignment showed that the amino acids involved in the interaction between DHHC9 and GCP16 were highly conserved in DHHC14 and 18. The team further validated that both DHHC14 and 18 need to be co-expressed with GCP16 in cells to obtain stable proteins, and both DHHC14-GCP16 and DHHC18-GCP16 complexes can catalyze palmitoylation of HRAS and NRAS. Knocking down DHHC9, 14, or 18 in HEK293T cells can reduce palmitoylation levels of NRAS and affect the subcellular localization of NRAS.
Related Products and Services
Protein Expression and Purification Services
Reference
Yang, A., Liu, S., Zhang, Y., Chen, J., Fan, Y., Wang, F., Zou, Y., Feng, S., Wu, J., & Hu, Q. (2024). Regulation of RAS palmitoyltransferases by accessory proteins and palmitoylation. Nature Structural & Molecular Biology, 1-11. https://doi.org/10.1038/s41594-023-01183-5