During antiretroviral therapy, the persistent presence of HIV-1 may be due to its establishment of a long-lived virus library in quiescent immune cells. Recently, an article titled “TGF-β blockade drives a transitional effector phenotype in T cells reversing SIV latency and decreasing SIV reservoirs in vivo” was published in the international journal Nature Communications. In the research report, scientists from institutions such as Northwestern University in the United States used HIV-1 infection models to explore how inhibiting immune cell signaling pathways combined with antiretroviral therapy can promote the body’s immune response and help reduce virus persistence.
The relevant research results may have the potential to improve scientists’ understanding of the underlying mechanisms behind HIV-1 evasion and attack on the host body’s immune system, while also developing new therapeutic strategies to combat this devastating disease.
Our Featured Products
| Cat. No. | Product Name | Source (Host) | Species | Tag |
| TGFB1-120H | Active Recombinant Human TGFB1 | CHO | Human | N/A |
| TGFB1-576M | Active Recombinant Mouse, Rat TGFB1 protein | HEK293 | Mouse, Rat | N/A |
| TGFB1-509H | Recombinant Human TGFB1 protein, Fc-tagged | HEK293 | Human | Fc |
| TGFB1-489H | Active Recombinant Human TGFB1 protein, His-tagged | HEK293 | Human | His |
| TGFB1-3209H | Recombinant Human TGFB1, His-tagged | E.coli | Human | His |
| TGFB1-429H | Recombinant Human TGFB1 Protein, His-tagged | E.coli | Human | His |
| TGFB1-126H | Recombinant Human Transforming Growth Factor, beta 1 | E.coli | Human | N/A |
| TGFB1-96B | Bovine Transforming Growth Factor beta-1 Reference standard | Bovine | N/A |
According to data from the CDC in the United States, approximately 1.2 million people are currently infected with HIV, with particularly high infection rates among male and black individuals. Although scientists are currently unable to cure HIV infection, antiretroviral therapy (ART) can help suppress virus replication and infected immune cells, thereby weakening the immune system function of patients and helping to improve their quality of life and prolong their lifespan.
Although the therapy has achieved some success, it must be taken by patients for a lifetime. HIV infection can persist and evade the body’s immune response through the “viral reservoirs”, and this situation occurs during the treatment period when the virus continues to exist in immune cells and remains latent.
In previous studies, researchers investigated the effect of cytokine TGF-β on the persistence of HIV-1. TGF-β can be released at high levels in HIV-1 patients. A study published in the JCI Insight journal in 2022 showed that TGF-β can inhibit the reactivation of HIV in latent infected immune cells, and blocking TGF-β signaling may promote the reactivation of retroviruses and enhance the body’s immune response.
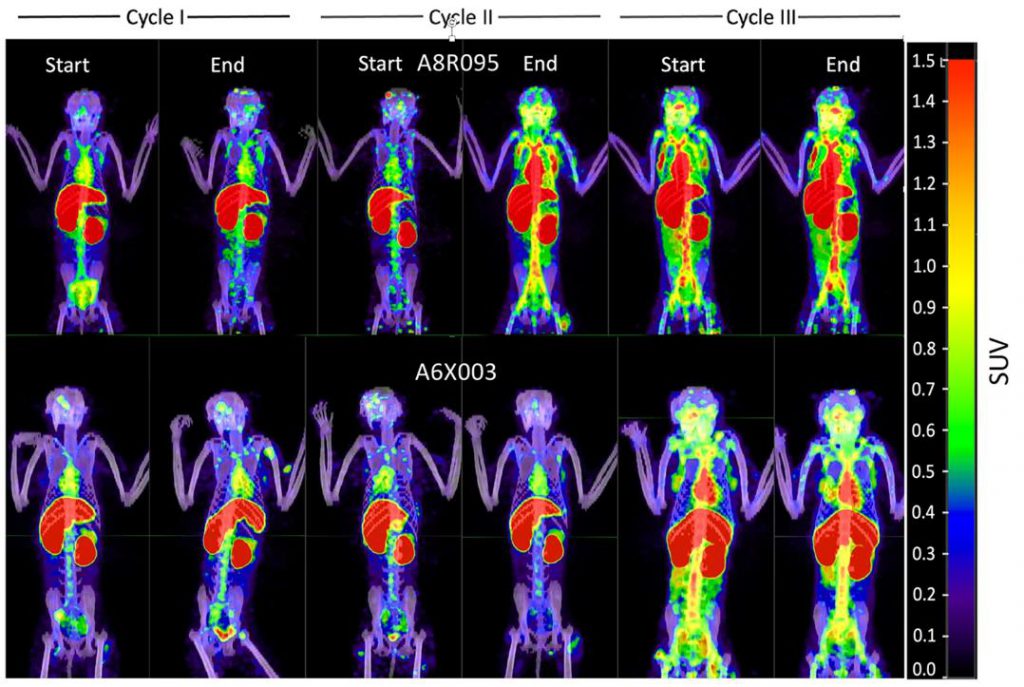
In the current research, researchers aim to elucidate the mechanisms behind promoting the HIV-1 virus library and better understand the effects of an inhibitor of TGF – β on immune cells infected with HIV. Martinelli said that the strategy they are working on developing is to use these drugs to replicate the virus. In this article, they use single-cell RNA sequencing and transcriptomics techniques to study the immune cells of non-human primate models infected with the simian immunodeficiency virus (SIV), a retrovirus that can cause persistent infections in non-primates. This type of model can utilize antiretroviral therapy and subsequent doses of galunisertib (TGF-β inhibitor) for treatment.
Researchers have found that this therapeutic strategy can promote the reactivation of the HIV-1 virus and reverse its latency, and this method can also enhance the immune response in CD4 T cells, other immune cells, and lymph nodes. This may be the beauty of this strategy, as it not only allows the virus to expose itself, but also enhances the host’s immune response, allowing immune cells to kill cells infected by the virus, which is essentially the reason for reducing the virus pool.
Researchers hope that TGF-β inhibitors can help control the viral load of HIV-1 when combined with antiretroviral therapy, ultimately allowing patients to discontinue antiretroviral therapy without increasing the risk of disease. However, further research is needed by later researchers to understand the impact of this on the persistence of HIV.
At present, researchers are working hard to better understand the mechanisms involved, especially focusing on events that occur in patient tissues. They have done a lot of work in searching for methods to cure HIV, but their focus has always been on what happens in the blood, as studying blood is relatively easier. However, with the support of these new technologies, researchers can truly observe events occurring in organizations and conduct larger-scale controlled studies.
In summary, the research results of this article indicate that a clinical research stage TGF-β Inhibitor – galunisertib may reverse the incubation period of primate immunodeficiency virus and reduce its virus pool by driving T cells to transition to effector phenotype, while also enhancing the host’s immune response without producing toxicity.
Related Products and Services
Protein Expression and Purification Services
Glial Cell-derived Neurotrophic Factor (GNDF) Subfamily
Reference
Kim, J., Bose, D., Araínga, M. et al. TGF-β blockade drives a transitional effector phenotype in T cells reversing SIV latency and decreasing SIV reservoirs in vivo. Nat Commun 15, 1348 (2024). doi:10.1038/s41467-024-45555-x