The immune system uses its mitochondria to self-stimulate innate and adaptive responses against infection. Reactive oxygen species (ROS), immunogenic mitochondrial DNA (mtDNA) and even whole mitochondria are mobilized locally in a delicate balance, generating hotspots of inflammatory action. When the normally limiting feedback of these processes is disrupted, detrimental autoimmune responses often arise.
A common sign of an abnormal immune system is the presence of antimitochondrial antibodies (AMA) in the blood. For example, in systemic lupus erythematosus (SLE), AMAs that target multiple mitochondrial compartments can be found. Some AMAs target proteins normally found in the outer mitochondrial membrane, while others target mtDNA. One question that arises naturally is how the immune system finds mtDNA released from mitochondria, given that mtDNA is normally located inside the mitochondrial matrix.
To address this issue, researchers from the National Heart, Lung, and Blood Institute have found that released mtDNA can cause lupus. In brief, when mitochondria are subjected to stress in multiple ways, mtDNA breaks into fragments that then bind to voltage-dependent anion channels (VDACs) in the outer mitochondrial membrane. This causes multiple VDAC monomers to come together and form a meta-pore in their middle through which mtDNA can escape. Once in the cytoplasm, various nonspecific sensing proteins, including the Toll receptor against single-stranded DNA and the GAS-STING pathway against double-stranded DNA, trigger a mature type I interferon (IFN) response. The relevant findings were recently published in Science under the title “VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease”.
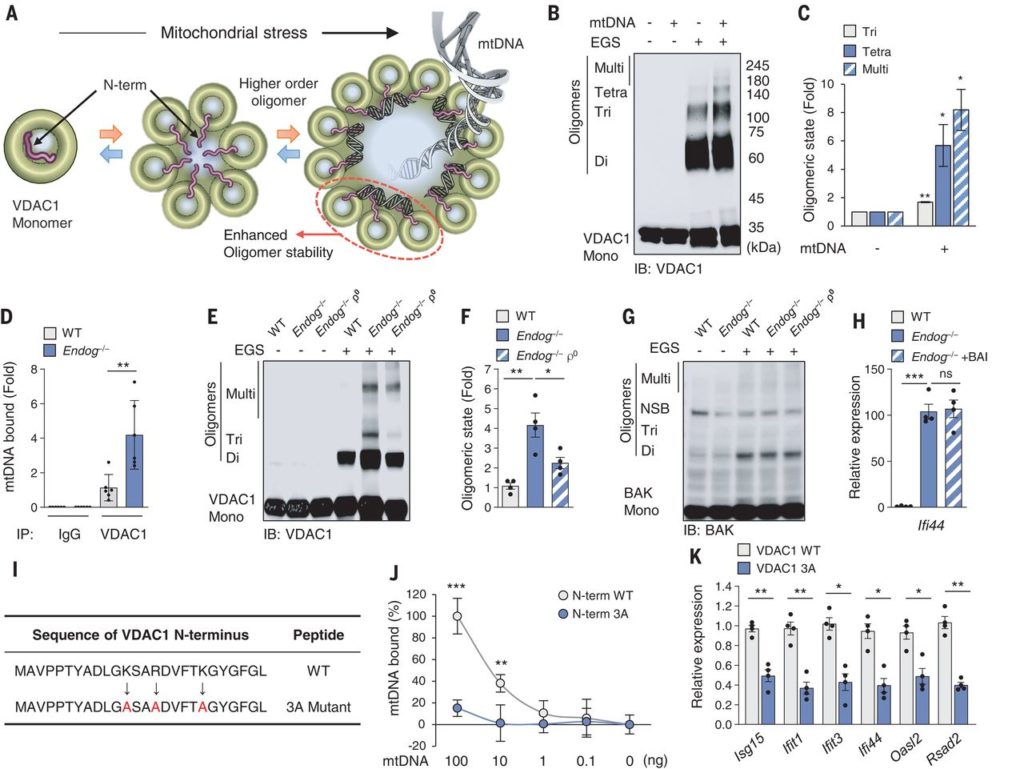
Each VDAC monomer itself contains a highly regulated channel that allows key molecules of different sizes and charges to pass in either direction based on the current membrane potential. Complete elimination of VDAC function does not work in higher eukaryotes. Fortunately, these researchers found that blocking only one of the channel forms, VDAC1—with the oligomerization inhibitor VBIT-4 abolished immune activation leading to lupus-like symptoms.
Discoid lupus erythematosus is the cutaneous form of lupus that is frequently associated with systemic lupus erythematosus. As a universal therapeutic strategy, interference with mtDNA release alone may not result in complete clearance of all AMA from the patient. However, this approach may be more useful for other forms of lupus, such as lupus nephritis, because all AMAs found appear to target double-stranded mtDNA.
Other types of autoimmune diseases, such as those affecting the hepatic bile ducts, may also be associated with AMA. Primary biliary cholangitis and primary sclerosing cholangitis are two diseases characterized by different forms of autoantibodies. Sclerosing cholangitis is associated with antinuclear antibodies (ANA). On the other hand, patients with biliary cholangitis have AMAs that target lipoates containing the E2 subunit of the pyruvate dehydrogenase complex. In addition, these patients often also have antibodies targeting enzymes such as sulfite oxidase and glycogen phosphorylase associated with liver mitochondria.
In the present context, it is unclear how and where these specific types of antibodies are generated. This immunogenic E2 subunit usually floats inside the mitochondrial matrix together with mitochondrial DNA and does not habitually escape through any channel. It has been postulated that abnormal fragments resulting from mitochondrial degradation by dying cells may help to promote autoantibody formation.
In an attempt to understand the production of AMA, an outstanding question that these researchers have hitherto ignored is how mtDNA travels through the inner mitochondrial membrane to the outer membrane VDAC. Peggy Crow of the Hospital for Special Surgery in New York, USA, points out that although the exact answer is unclear, imaging studies have shown another pore system that acts in parallel with VDAC. These so-called “BAK/BAX macropores” allow the mitochondrial inner membrane to protrude into the cytoplasm, permeabilize and transport matrix components including mtDNA.
So far, these researchers have not talked much about reactive oxygen species in mitochondrial inflammation. Another of their findings has linked oligomerization of VDAC1 channels and ROS. More specifically, they found that a molecule called lipoxstatin-1 protects cells from reactive oxygen species by reducing the levels of VDA1 and restoring the levels of the enzyme GPX4.
Gpx4 is a unique selenium-utilizing form of glutathione peroxidase that specifically protects lipids in the cell membrane from oxidative damage. A unique form of apoptosis, ferroptosis, occurs throughout the cell when GPx4 is damaged. By preventing oligomerization of VDAC1 but not VDAC2 or VDAC3, they found that liproxstatin-1 could short-circuit the iron death pathway.
Importantly, lipoxstatin-1 can also block mitochondrial contraction, the reduction and disruption of cristae within mitochondria, and the rupture of other mitochondrial membranes leading to iron death. Gpx4 deficiency is not an autoimmune disease, but a disease characterized by uncontrolled ROS damage and iron death occurring within an extremely restricted cell population. The disease is so rare that a recently diagnosed patient is the only person in the world with the mysterious disease.
Reference
1.Jeonghan Kim et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science, 2019, doi:10.1126/science.aav4011.
2.Mary K. Crow. Mitochondrial DNA promotes autoimmunity. Science, 2019, doi:10.1126/science.aaz9308.
3.Know thy mitochondria: Autoimmunity to organelles and their DNA
https://medicalxpress.com/news/2020-01-thy-mitochondria-autoimmunity-organelles-dna.html