In a new study, researchers from research institutions such as the University of California, Los Angeles, discovered that advanced genome editing technology may be able to be used for a rare and fatal genetic disease, D3 delta severe combined immunodeficiency, CD3δ SCID, for a one-time treatment. The relevant research results were published online in Cell, with the title “Human T cell generation is restored in CD3δ severe combined immunodeficiency through adenine base editing”.
CD3δ SCID is caused by a mutation in the CD3D gene, which blocks the production of CD3δ proteins that are required for hematopoietic stem cells to normally produce T cells. No T cells, infants with CD3δ SCID are unable to resist infection and, if untreated, often die within the first two years of life. Currently, bone marrow transplantation is the only available treatment, but this method has significant risks.
In this new study, the authors discovered a new genome editing technique called base editing that can correct for CD3δ SCID mutations in hematopoietic stem cells and restore their ability to produce T cells. This potential treatment is the result of a laboratory collaboration between Dr. Donald Kohn and Dr. Gay Crooks at the University of California, Los Angeles.
Kohn’s laboratory has previously successfully developed gene therapy for several immune system defects, including other forms of severe combined immunodeficiency (SCID). At the request of Dr. Nicola Wright, a pediatric hematology and immunology expert at the Children’s Hospital Research Institute in Alberta, Canada, he and his colleagues turned their attention to CD3 δ SCID, Wright was looking for better treatment options for her patients at the time.
CD3 δ SCID is common in Mennonite communities that immigrate between Canada and Mexico. Wright said, “Due to the lack of SCID screening for newborns in Mexico, I often see some infants diagnosed late and seriously ill after returning to Canada.”
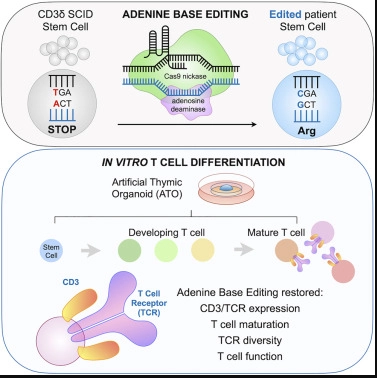
When Kohn made Wright’s request to his laboratory, Grace McAuley, a research assistant who joined Kohn’s laboratory at the end of his senior year at the University of California, Los Angeles, came forward with a bold idea. “McAuley suggested that we try base editing, which is a very new technology that my lab has never tried before,” Kohn said.
Base editing is a form of ultra-precise genome editing that enables scientists to correct single base mutations in DNA. DNA consists of four bases – A, T, C, and G -; these base pairs form “rung” in the DNA double helix trapezoidal structure. Other gene editing platforms, such as CRISPR-Cas9, cut two strands of chromosomes to change DNA, while base editing uses chemical methods to change one DNA base into another (such as changing A into G), keeping chromosomes intact.
McAuley said, “At first I had a very steep learning curve, and base editing didn’t work at that time. But I continued to move forward. My goal was to help promote this therapy to the clinic as quickly as possible.”
McAuley contacted David Liu of the Broad Institute (inventor of base editing) for advice on how to evaluate the safety of the technology for this particular use. Finally, McAuley identified a base editor that could efficiently correct mutations in pathogenic genes.
Because this disease is extremely rare, obtaining hematopoietic stem cells from patients for this new study is a major challenge. After Wright provided the donation of hematopoietic stem cells from a CD3δ SCID patient, who was undergoing a bone marrow transplant, the project has been promoted. The base editor corrected for an average of nearly 71% of the patient’s hematopoietic stem cells in three laboratory experiments.
Next, McAuley collaborated with Dr. Gloria Yiu, a clinical lecturer in rheumatology at the University of California, Los Angeles, to test whether the corrected hematopoietic stem cells can produce T cells. Yiu uses an artificial thymoid organ, which is a stem cell-derived tissue model developed by Crooks Laboratories to simulate the human thymus environment. When these calibrated hematopoietic stem cells are introduced into artificial thymic organs, they produce fully functional mature T cells.
“Because artificial thymic organs can effectively support the development of mature T cells, they are an ideal system, indicating that base editing of patient hematopoietic stem cells can repair defects in this disease,” Yu said.
As a final step, McAuley studied the lifespan of calibrated hematopoietic stem cells by transplanting them into mice. Four months after transplantation, these corrected hematopoietic stem cells still exist, indicating that base editing has corrected for mutations in genuine, self-renewing hematopoietic stem cells. These research results indicate that the corrected hematopoietic stem cells can exist for a long time and produce the T cells needed by patients for a healthy life.
These authors are studying how to bring this new approach to CD3δ SCID infants in clinical trials in Canada, Mexico, and the United States.
The treatment described in this study is only used for preclinical testing, has not been tested on humans, and has not been approved by the US Food and Drug Administration (FDA) for safe and effective use in humans. The technology was filed with a patent application, and Kohn and McAuley were listed as co-inventors.
Related Product & Service
Pharmaceutical Product Release Testing
Downstream Processing Development
Reference
Grace E. McAuley et al. Human T cell generation is restored in CD3δ severe combined immunodeficiency through adenine base editing. Cell, 2023, doi:10.1016/j.cell.2023.02.027.