In a new study, Dr. David Jacques, a medical researcher at the University of New South Wales in Australia, and his team discovered how the human immunodeficiency virus (HIV) breaks through the nucleus to establish infection, a discovery that goes beyond the scope of HIV biology. The relevant research results were published online in the journal Nature, with the title “The HIV capsid mimics karyopherin engagement of FG-nucleoporins”.
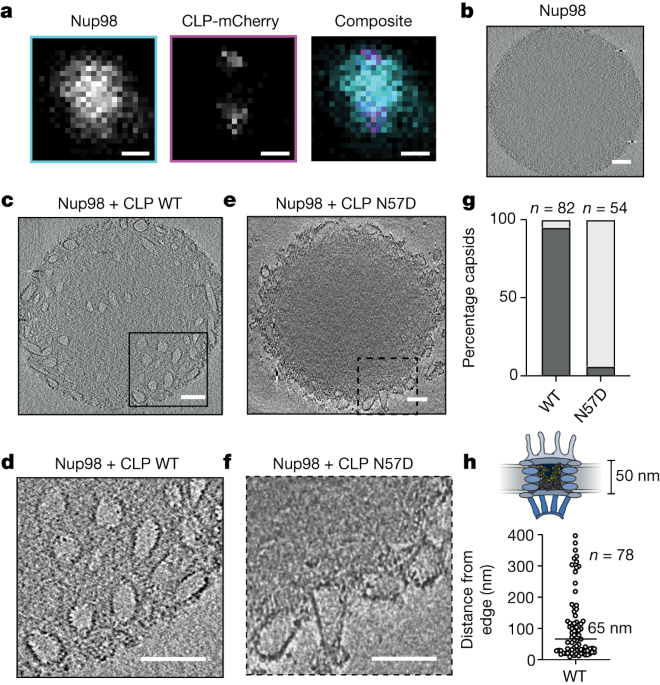
To infect cells, HIV must enter the target cell and reach the nucleus located at the center of the cell, where it produces enough copies of the genetic code to infect other cells. To safely complete this task, this virus constructs a protective protein coat called a capsid to resist the host’s immune defense system set up to eliminate it. Before this, it has been a mystery how a complete capsid passes through the nuclear pores embedded in the nuclear membrane and enters the nucleus. This new study reveals how the HIV capsid enters the nuclear pore barrier channel.
Our Featured Products
| Cat.No. | Product Name | Source | Species | Tag |
| gp120-26H | Active Recombinant HIV-1 gp120 | E.coli | HIV | N/A |
| gp160-330V | Active Recombinant HIV gp160 Protein, Fc-tagged | HEK293 | HIV | Fc |
| RT-89H | Active Recombinant HIV-1 RT | E.coli | HIV | N/A |
| gp160-314V | Recombinant HIV gp160 Protein, His-tagged | HEK293 | HIV | His |
| gag-356V | Recombinant HIV gag Protein, His-tagged | E.coli | HIV | His |
| gp160-636V | Recombinant HIV gp160 (subtype CRF01_AB, strain 07JP_NMC716_clone_01) Protein, His & MBP-tagged | E.coli | HIV | His/MBP |
| gp160-724V | Recombinant HIV gp160 Protein, Fc-tagged | HEK293 | HIV | Fc |
| gag-725V | Recombinant HIV gag Protein, His-tagged | E.coli | HIV | His |
Dr. Jacques said, “The nuclear pore complex is composed of a combination of multiple proteins. Although small molecules can enter and exit the nucleus through the nuclear pore complex, the entry and exit of large-volume goods are limited. Larger proteins need to bind with the nuclear transporter – chaperone protein – to pass through this multi-layer molecular gate, known as the nuclear pore.”
The Jacques team found that although the capsid of HIV is a thousand times larger than the molecule that passes through the nuclear pore barrier channel, it can pass through the nuclear pore barrier channel without a chaperone protein.
In the nucleus, chaperones – also known as nuclear transporters – bind to barrier proteins in the nuclear pore complex, allowing them to carry payloads into each layer of the nuclear pore barrier channel. Large-volume structures without companion proteins are excluded from this entrance as they cannot connect with barrier proteins in the nuclear pore.
However, the capsid of HIV has evolved to interact with barrier proteins in the same way as host partner proteins. Jacques said, “One theory in this field suggests that HIV hijacks the host’s chaperone protein to enter the nucleus. However, our research results indicate that HIV does not require a chaperone protein because it is its own chaperone protein. It’s like the HIV capsid has learned the secret handshake method of allowing entry into the forbidden zone by simulating chaperone proteins.”
Dr. Claire Dickson, co-first author of the paper, said, “People have made hypotheses about how HIV capsids pass through this selective barrier channel. Our research is truly starting to directly address this issue, which is exciting for me.”
These findings are attributed to a single-molecule approach previously developed by the Jacques team, which allows them to systematically screen proteins in nuclear pore complexes to identify those that can interact with the intact HIV capsid.
Dr. Sophie Hertel, the first author of the paper, said, “I am proud to be able to overcome some major challenges in this project and have a significant impact on the field of AIDS research by helping people better understand the process of HIV entering the nucleus.”
These authors believe that the molecular knowledge gained in this study on the interaction between hosts and pathogens not only reveals the details of the HIV lifecycle. This mechanical knowledge may also be used for other applications, including gene therapy.
Dr. Jacques said, “HIV is one of the most extensively studied pathogens, but there is still a lot we can learn from it. HIV has its uniqueness: it can enter the nucleus without damaging it, rather than waiting for cell division like other viruses. Our observations have given us insights and allowed us to think about how to deliver molecular cargo into the nucleus.”
Related Products and Services
Protein Expression and Purification Services
Reference
C. F. Dickson et al. The HIV capsid mimics karyopherin engagement of FG-nucleoporins. Nature, 2024, doi:10.1038/s41586-023-06969-7.