Cell-Free In Vitro Protein Expression
The rising need for rapid, scalable protein expression systems in biotechnology—fueled by drug discovery, synthetic biology, and precision medicine—has exposed limitations in traditional cell-based methods, such as slow timelines and incompatibility with toxic or complex targets. Cell-free in vitro protein synthesis offers a streamlined alternative, enabling efficient production of functional proteins, including membrane proteins and post-translationally modified variants, within hours.
Creative BioMart provides cutting-edge cell-free expression solutions, utilizing high-yield protocols and customizable workflows to accelerate research and industrial applications. Our expertise in recombinant antibody synthesis , membrane protein production , and custom expression systems ensures reliable results for structural biology, diagnostics, and beyond.
Optimize your protein studies with speed and precision — connect with Creative BioMart today!
Background
What is Cell-Free In Vitro Protein Expression?
Cell-free in vitro protein expression can be performed in so-called "coupled" systems running RNA transcription and protein translation at the same time in the same reaction mixture. It is a preferable choice for many applications in protein research including options for protein labeling and the expression of difficult-to-express proteins like membrane proteins and multiple protein complexes. Only two basic conditions are required to establish the system: the template encoding the target protein and a reaction solution containing the necessary transcriptional and translational molecular machinery.
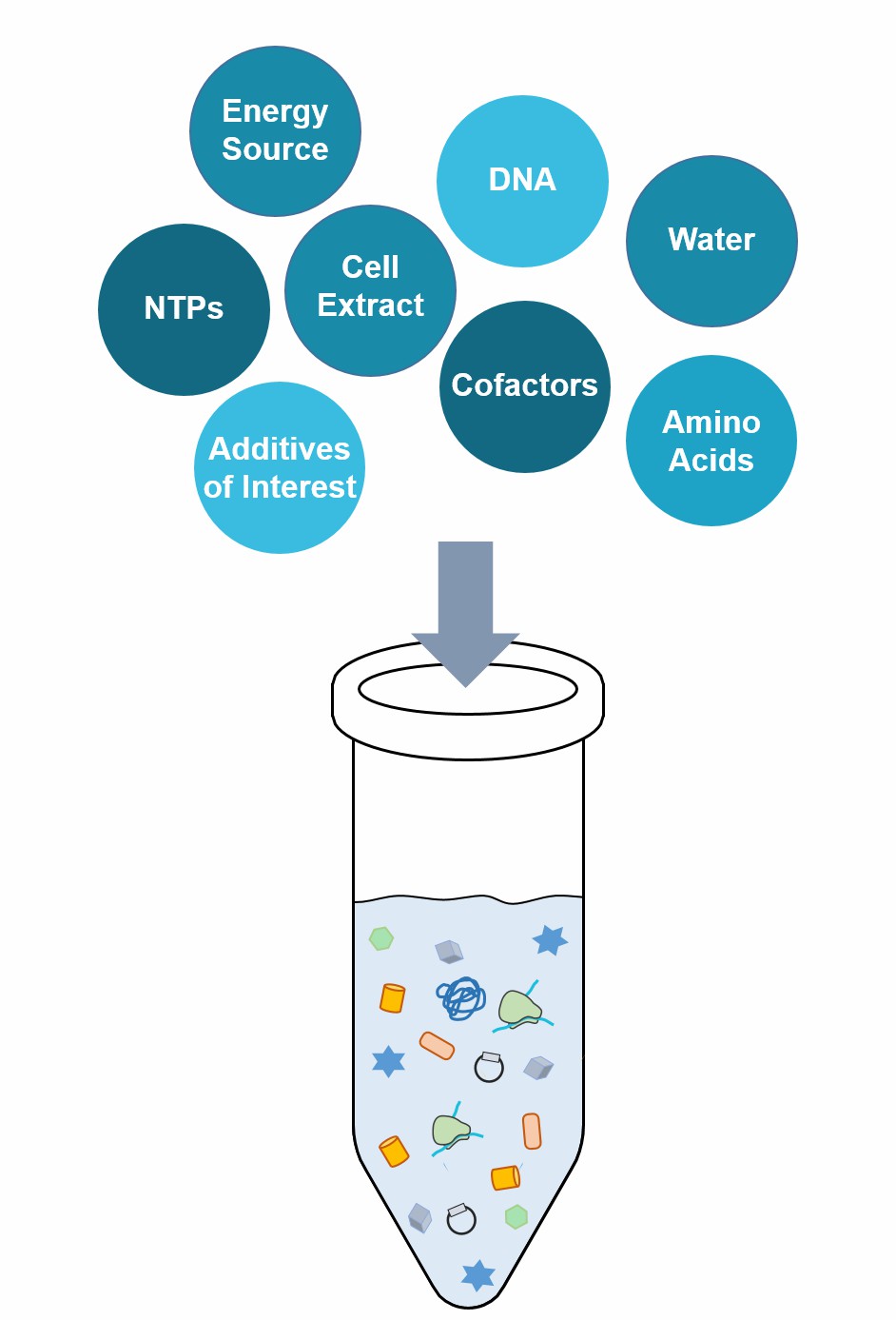
Cell Extracts Supply All Or Most of The Molecules of The Reaction Solution
RNA polymerases for mRNA transcription
Ribosomes for polypeptide translation
tRNA and amino acids
Enzymatic cofactors and an energy source
Cellular components essential for protein folding
Advantages
- Able to express proteins that are toxic to host cells
- High throughput, can express several different proteins simultaneously
- Reaction conditions are easy to change, allowing for protein modification control
- Ability to express different types of proteins
- Capable of producing cytotoxic proteins
- Simple operation and time saving
Applications
- Accelerating Biomedical Research Creative BioMart’s solutions enable rapid protein production for structural biology (e.g., X-ray crystallography, NMR) and functional studies, particularly for challenging targets like membrane proteins or toxic variants. We support high-throughput drug screening by synthesizing functional receptors, enzymes, and disease-associated proteins within hours, streamlining early-stage drug discovery and target validation.
- Industrial Biotechnology & Enzyme Engineering We deliver scalable, cost-effective recombinant protein production for industrial enzymes, biosensors, and biocatalysts, catering to sectors like biofuels, agriculture, and bioremediation. Our expertise in enzyme optimization allows tailored engineering of stability, activity, or substrate specificity to meet specific industrial demands.
- Advancing Therapeutics & Vaccine Development Our platform accelerates therapeutic protein synthesis, including antibodies, cytokines, and growth factors, with minimized batch variability for preclinical and clinical applications. For vaccine development, we enable rapid antigen production to address emerging pathogens or personalized oncology targets, ensuring agility in responding to global health needs.
- 4. Academic Research & Education We provide simplified, cell-free systems for teaching core concepts in molecular biology, eliminating the need for complex cell culture infrastructure. Academic labs leverage our services to express difficult-to-produce proteins (e.g., post-translationally modified or toxic variants), fostering innovation in basic and applied research.

Service Details
1
Gene Synthesis Plus Codon Optimization
- Leading codon optimization system
- Analysis and optimization of rare codons, codon bias and other factors
Timeline: 1-2 weeks
2
Vector Construction
- Clone target gene into expression vector
- Plasmid sequencing
- Plasmid preparation
Timeline: 1 week
3
Protein Expression and Purification
- Expression condition optimization
- Protein expression evaluation
- Various protein purification methods
Timeline: 1-2 weeks
4
Optional Services
- Secondary purification
- Tag removal
- Endotoxin removal
- Activity assay
- Protein analysis
- Other more
Timeline: 1-2 weeks
5
Quality Control
- SDS-PAGE
- Western blot
- Mass spectrometry
Timeline: <1 week
Choosing the Right Cell-Free Expression System
Creative BioMart offers a wide variety of cell-free in vitro systems. Selection of a cell-free system should be according to the biological nature of the protein, application and the template used for protein expression. Our team can guide you through the process of the cell-free expression if you would like to discuss more about your project.
| System | Advantages | Disadvantages |
|---|---|---|
| E.coli Extract |
|
|
| Wheat Germ |
|
|
| Insect Cell Lysate |
|
|
| Rabbit Reticulocytelysate |
|
|
| Human Cell Lysate |
|
|
Service Delivery & Submission Guidelines
-
- Submission Requirements
- Sample Information : Provide target gene sequences (plasmid, linear DNA, or PCR product) with preferred cloning vectors or specifications (e.g., His-tag, fluorescent labels).
- Expression Conditions : Specify desired parameters (e.g., temperature, reaction time, post-translational modifications) or request optimization support from our team.
- Application Details : Clarify the intended use (e.g., structural biology, drug screening) to guide protein purity, yield, and formulation.
-
- Delivery Details
- Turnaround Time : Standard delivery within 4-6 weeks . Urgent projects prioritized.
- Output Formats : Receive purified proteins, crude lysates, or lyophilized products, accompanied by QC reports (SDS-PAGE, Western blot, mass spectrometry).
- Scalability : Services include small-scale research batches (μg-mg) to large-scale industrial production (g+), tailored to your needs.
Why Choose Us?
- Technical Expertise. Specializing in cell-free protein synthesis for over a decade, we tackle complex targets like membrane proteins and toxic variants with proven success.
- Fast & Scalable Delivery. Achieve results in 4-6 weeks with flexible scaling—from µg research batches to industrial g+ production.
- Custom Solutions. Tailor workflows for His-tagged proteins, lyophilized antigens, or other specific needs via flexible vector and protocol design.
- Quality Guaranteed. All deliverables include QC reports (SDS-PAGE, activity assays) and ISO-certified consistency for therapeutic or diagnostic use.
- Responsive Support. Get end-to-end guidance and real-time updates from our experts, ensuring alignment with your goals.
- Trusted Partner. Relied on by global biotech and academic leaders for enzyme engineering, vaccine R&D, and synthetic biology breakthroughs.
Case Study
Case 1: Combining In Vitro Folding with Cell-Free Protein Synthesis for Membrane Protein Expression
Research Background : CD1E, essential for glycolipid antigen presentation on T cells, is challenging to produce via cell-based systems due to low yields and misfolding, hindering immunological research.
Methods : CD1E was synthesized using an E. coli CFPS system with detergents, purified via Ni²⁺ affinity chromatography (>90% purity). B2M, predicted by STRING as a partner, was similarly produced. Binding was validated by SPR.
Results : CFPS generated functional CD1E and B2M with confirmed interaction (KD = 2.3 nM). The method avoided cell-based pitfalls (e.g., cytotoxicity) and accelerated production.
Conclusion : This cell-free protein synthesis strategy enables scalable, functional production of CD1E, advancing studies on antigen presentation and therapeutics for membrane protein targets.

Fig1. Effect of three different reaction systems on the expression of CD1E protein by SDS-PAGE. (Tang, et al. , 2023)
Research Background: Traditional cell-free systems rely on natural tRNAs, limiting flexibility for genetic code engineering and synthetic biology applications like artificial cell development. A fully customizable tRNA system is critical to overcome these constraints.
Methods : A reconstituted PURE system was equipped with 21 unmodified in vitro transcribed tRNAs (iVTtRNAs). Genetic code rewriting was tested by altering iVTtRNA composition. Modified nucleotides were introduced to enhance yield and fidelity, comparing DHFR production to native tRNA reactions.
Results : The iVTtRNA system synthesized active proteins with redesigned genetic codes. Modified nucleotides boosted DHFR yields to 40% of native tRNA levels (30°C) while improving decoding accuracy.
Conclusion : This cell-free tRNA reconstitution platform enables precise genetic code manipulation, advancing synthetic biology and protein engineering. It bridges gaps in studying decoding mechanisms and designing artificial self-replicating systems.

Fig2. Dependency of tRNA concentration on the yield of synthesized DHFR. (Hibi, et al. , 2020)
Case 3: Cell-Free Synthesis of Brochosomin Proteins for Advanced Nanomaterial Engineering
Research Background : Brochosomes, natural nanostructures with superhydrophobic/antireflective traits, are hindered by poor brochosomin subunit understanding and redox sensitivity due to critical disulfide bonds. Traditional methods fail to address these challenges.
Methods : Brochosomins were synthesized via cell-free gene expression (CFE), optimizing redox buffers, temperature (25–30°C), and disulfide isomerase levels. Solubility and aggregation were analyzed using dynamic light scattering (DLS) and transmission electron microscopy (TEM).
Results: Achieved soluble brochosomin yields of 341 ± 30 μg/mL. DLS/TEM revealed subunit-specific aggregation patterns, enabling controlled nanostructure assembly. CFE bypassed redox instability issues seen in cell-based systems.
Conclusion : This cell-free protein synthesis approach unlocks scalable production and analysis of redox-sensitive brochosomins, accelerating bioinspired nanomaterials design. The platform extends to studying other complex, disulfide-dependent protein assemblies.

Fig3. Optimizations of CFE reactions were performed to reduce aggregation of brochosomin protein HV3. (Lay, et al. , 2025)
Customer Testimonials
-
"Needing rapid antibody fragment production, your cell-free protein synthesis delivered 10mg in 48 hours—60% faster than traditional systems. Crucial for accelerating our ADC pipeline."
-Dr. Emily Chen, Director of Manufacturing Operations | Global Biologics Manufacturer
-
"Your cell-free platform expressed toxic proteases with 90% solubility, bypassing cell viability issues. A breakthrough for difficult-to-express protein services in industrial biocatalysis."
-Mr. James O’Connor, Head of R&D | Industrial Enzyme Solutions
-
“Produced glycosylated viral antigens without mammalian cells. Saved 5 weeks on process development. A game-changer for scalable vaccine antigen manufacturing.”
-Dr. Hiroshi Tanaka, CMC Lead | Vaccine Development Corp.
-
"Ordered 50+ codon-optimized variants for high-throughput screening. Your cell-free expression kits eliminated cloning delays, slashing project timelines by 40%."
-Ms. Fatima Al-Mansoori, Production Manager | Synthetic Biology Innovators
-
"On-demand synthesis of patient-specific neoantigens enabled Phase I trials in 3 months. Unmatched flexibility for personalized therapeutic protein production."
-Dr. Lucas Weber, VP of Bioprocessing | Precision Medicine Group
-
"Batch-to-batch consistency met GMP-grade specs (<0.1 EU/µg endotoxin). Ideal for clinical-stage cell-free protein services requiring rigorous documentation."
-Ms. Anika Patel, QA/QC Director | Pharma Compliance Network
FAQs
-
Q: What types of proteins can be expressed using your cell-free in vitro protein expression systems?
A: Our systems can express a wide range of proteins, including membrane proteins, toxic proteins, large proteins, and those requiring post-translational modifications.
-
Q: How do I choose the right cell-free expression system for my protein?
A: Selection depends on the protein type, application, and template. We offer various systems ( E. coli , wheat germ, insect cell lysate, etc.) and can guide you to choose the best one.
-
Q: What are the advantages of using cell-free in vitro protein expression over traditional cell-based methods?
A: Cell-free systems offer rapid production, high throughput, ability to express toxic proteins, and ease of modification. They save time and are ideal for complex proteins.
-
Q: What quality control measures do you provide with your cell-free protein expression service?
A: We provide SDS-PAGE, Western blot, and mass spectrometry to ensure protein purity and integrity. Activity assays are also available for functional verification.
-
Q: Can your cell-free in vitro protein expression service be scaled up for industrial production?
A: Yes, our service is scalable from small research batches to large industrial quantities, with flexible options to meet your needs.
Other Resources
References:
- Tang Y.; et al . Cell-free protein synthesis of CD1E and B2M protein and in vitro interaction. Protein Expr Purif. 2023;203:106209.
- Hibi K.; et al . Reconstituted cell-free protein synthesis using in vitro transcribed tRNAs. Commun Biol . 2020;3(1):350.
- Lay CG.; et al . Cell-Free Expression of Soluble Leafhopper Proteins from Brochosomes. ACS Synth Biol . 2025;14(3):987-994.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions


