Matrix Metallopeptidases
SubCategories
Matrix Metallopeptidase—Product Overview
Matrix metallopeptidases (MMPs) are a family of zinc-dependent endopeptidases that play a critical role in extracellular matrix (ECM) remodeling, tissue repair and immune responses. These enzymes regulate key physiological and pathological processes, including wound healing, angiogenesis, and tumor progression. Dysregulation of MMP activity is associated with cancer metastasis, inflammatory diseases and fibrosis, making them important targets for therapeutic research. Creative BioMart offers a comprehensive range of recombinant MMP proteins and related products to support research in cancer biology, tissue remodeling, drug development and disease pathology.
Jump to Section
Product Families
-
Matrix Metallopeptidases
-
MMP Inhibitors
Background
MMPs are a family of zinc-dependent endopeptidases that degrade components of the extracellular matrix (ECM) and play a critical role in tissue remodeling, wound healing, and immune responses. These enzymes regulate various biological processes by degrading proteins such as collagen, elastin, and gelatin. MMPs are tightly controlled by tissue inhibitors of metalloproteinases (TIMPs) to maintain homeostasis. Dysregulation of MMP activity has been implicated in pathological conditions such as cancer metastasis, fibrosis and inflammatory diseases.
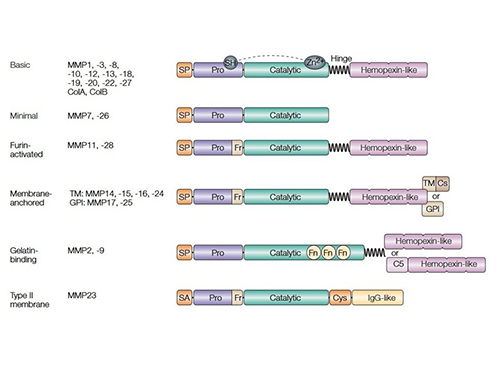
MMPs are classified into collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs based on substrate specificity and structural features. They share a conserved domain structure, including a catalytic zinc-binding domain, a pro-peptide domain that maintains enzyme latency, and a hemopexin-like domain for substrate recognition. Some MMPs are membrane-bound, while others are secreted as inactive zymogens that require proteolytic activation. Their structural diversity allows them to participate in a wide range of physiological and pathological processes.
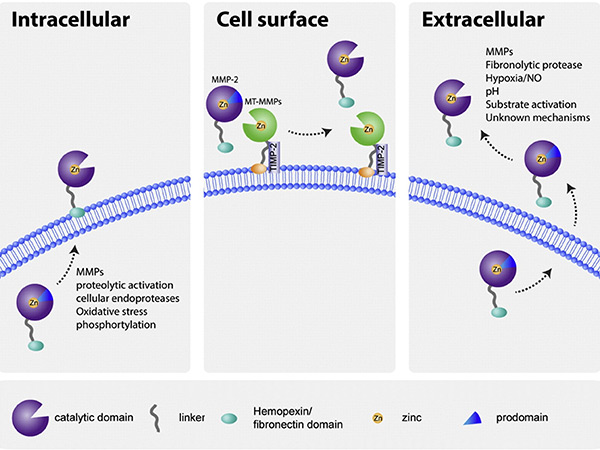
MMP activity is tightly regulated at multiple levels, including gene transcription, zymogen activation, and inhibition by TIMPs. They are initially synthesized as inactive proenzymes and activated by proteolytic cleavage, often involving other proteases or oxidative stress. TIMPs specifically bind to MMPs to prevent excessive ECM degradation. Disruption of this regulation can lead to various diseases such as chronic inflammation, arthritis and tumor progression, highlighting the importance of maintaining MMP balance in the cellular environment.
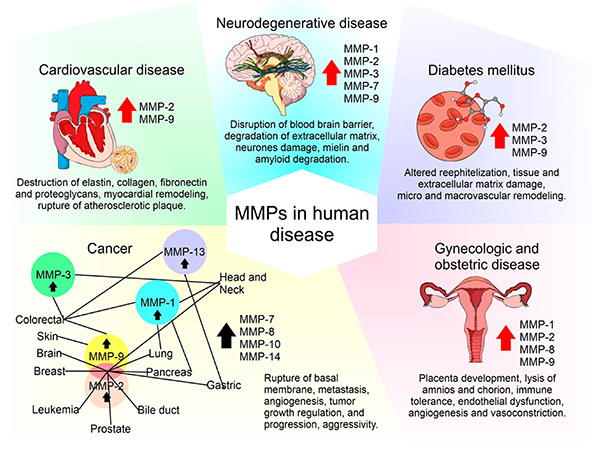
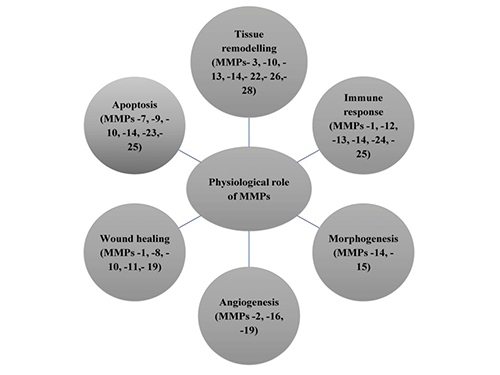
Aberrant MMP expression is associated with cancer, cardiovascular disease and neurodegenerative disorders. In cancer, MMPs facilitate tumor invasion and metastasis by breaking down ECM barriers. In cardiovascular disease, they contribute to plaque instability and aneurysm formation. Because of their involvement in disease progression, MMP inhibitors have been investigated as potential therapeutic agents. However, challenges such as specificity and side effects have limited clinical success, prompting ongoing research into targeted MMP modulation strategies.
Applications

Cancer Research
MMPs play a critical role in tumor progression by degrading extracellular matrix components, facilitating cancer cell invasion and metastasis. They are widely studied as biomarkers for cancer diagnosis and as potential therapeutic targets to inhibit tumor growth and spread, thereby aiding in the development of novel anticancer drugs.
Tissue Remodeling and Wound Healing
MMPs regulate extracellular matrix turnover, making them essential for tissue remodeling and wound healing. They are involved in cell migration, angiogenesis and tissue repair and are being investigated for therapeutic applications in chronic wounds, fibrosis and regenerative medicine.
Inflammatory and Autoimmune Diseases
Dysregulated MMP activity is implicated in inflammatory and autoimmune diseases such as arthritis and multiple sclerosis. MMP inhibitors are being investigated for their potential in controlling excessive tissue degradation and modulating immune responses to alleviate disease symptoms.
Neurological Disorders
MMPs influence blood-brain barrier permeability and neuroinflammation, playing roles in stroke, Alzheimer’s disease, and multiple sclerosis. Research on MMP inhibitors and modulators aims to develop treatments that prevent neurodegeneration and improve neural repair.
Cardiovascular Diseases
MMPs contribute to vascular remodeling, atherosclerosis, and hypertension by degrading arterial walls and influencing plaque stability. They serve as therapeutic targets and biomarkers in cardiovascular disease research, aiding in the development of new drugs for heart disease and stroke prevention.
Product Features
-
High Purity & Bioactivity: Our MMP proteins are produced using advanced purification techniques to ensure high purity, proper folding, and enzymatic activity for reliable research results.
-
Wide Selection of MMPs: We offer recombinant MMP proteins, including MMP-1, MMP-2, MMP-9, MMP-13, and more, covering key roles in cancer, inflammation, and tissue remodeling studies.
-
Multiple Expression Systems: Available in E. coli, mammalian, and insect cell systems, ensuring optimal post-translational modifications and activity for different research applications.
-
Validated for Various Applications: Suitable for use in biochemical assays, drug screening, enzyme activity studies, and disease modeling in areas like cancer research and regenerative medicine.
-
Custom Production Services: Tailored solutions including protein expression, purification, and activity validation to meet specific research and biopharmaceutical needs.
-
Bulk & OEM Supply: Scalable production options for large-scale research, pharmaceutical development, and contract manufacturing.
-
Application-Ready: Designed for studies in tumor metastasis, wound healing, neurodegenerative disorders, and cardiovascular diseases.
Case Study
Case 1: MMP1 Regulation by ZNF3 in CRC
Du et al., 2022. ZNF3 regulates proliferation, migration and invasion through MMP1 and TWIST in colorectal cancer.
Zinc finger protein 3 (ZNF3) is a transcription factor previously linked to breast cancer prognosis, but its role in colorectal cancer (CRC) was unclear. Recent studies show that ZNF3 is highly expressed in CRC tissues and cells, and its expression correlates with patient age. Overexpression of ZNF3 promotes CRC cell migration, while its knockdown significantly inhibits cell proliferation, migration, invasion, and induces G0/G1 phase cell cycle arrest. Silencing ZNF3 also reduces expression of EMT markers TWIST and MMP1. Overexpressing MMP1 and TWIST in ZNF3-knockdown cells restores cell proliferation and invasion, indicating that ZNF3 regulates CRC progression through MMP1 and TWIST.
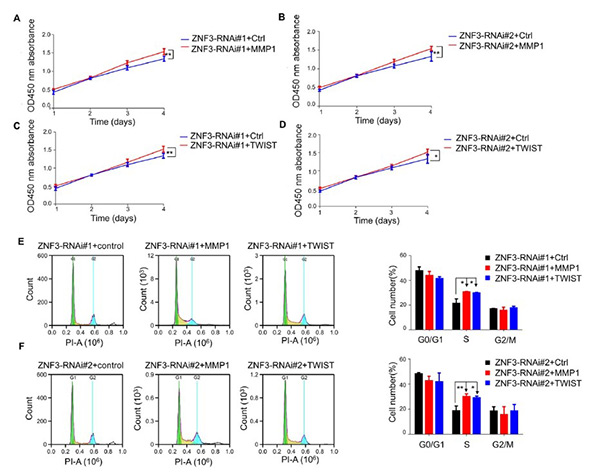
Figure 1. Overexpression of MMP1 and TWIST promotes the proliferation of ZNF3-knockdown SW480 cells (A, B) Effect of MMP1 on the proliferation of ZNF3-knockdown SW480 cells. (C, D) Effect of TWIST on the proliferation of ZNF3-knockdown SW480 cells. (E, F) Effect of MMP1 and TWIST overexpression on the cell cycle of ZNF3-knockdown SW480 cells. *P<0.05, and **P<0.01.
Case 2: EVs and MMP2 in Thyroid Cancer
Bravo-Miana et al., 2022. Extracellular vesicles from thyroid cancer harbor a functional machinery involved in extracellular matrix remodeling.
Extracellular vesicles (EVs) play a key role in cell-stroma interactions within the tumor microenvironment, with fibroblasts (Fb) contributing to tumor promotion in thyroid cancer. A study investigated how thyroid tumor cells (TPC-1, 8505c) and non-tumor cells (NThyOri) interact with fibroblasts to release EVs with specific proteomic profiles. EVs from tumor-Fb co-cultures exhibited markers related to extracellular matrix (ECM) remodeling, enhancing EV functionality. These EVs activated MMP2, facilitating ECM degradation and potentially promoting tumor progression. Additionally, fibroblasts internalized more EVs from tumor cells than non-tumor cells. The study also identified MMP2 interactors that could distinguish tumor-derived EVs from non-tumor ones, providing insights into EV-based cell communication in thyroid cancer.
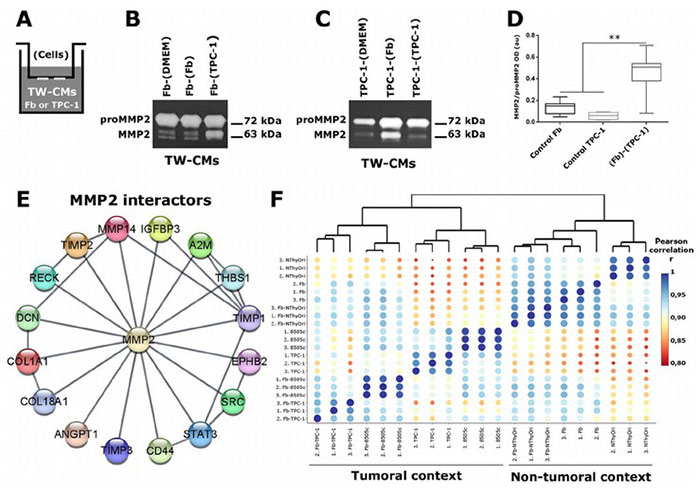
Figure 2. BMP7 increases MMP2 expression and activity in SW1353 cells.
FAQs
-
Q: What are Matrix Metallopeptidases (MMPs), and why are they important?
A: MMPs are a family of zinc-dependent proteases that degrade extracellular matrix components, playing crucial roles in tissue remodeling, wound healing, cancer metastasis, and inflammatory diseases. -
Q: What types of MMP proteins do you offer?
A: We provide a wide range of recombinant MMPs, including MMP-1, MMP-2, MMP-7, MMP-9, and more, suitable for various research applications in cancer biology, cardiovascular studies, and regenerative medicine.
-
Q: How should I store MMP proteins for optimal stability?
A: Most MMP proteins should be stored at -80°C upon arrival. Avoid repeated freeze-thaw cycles to maintain their activity and stability. Specific storage recommendations are provided with each product.
-
Q: Are your MMP proteins active or inactive?
A: We offer both active and pro-form (inactive) MMPs to accommodate different experimental needs. Please check the product specifications or contact us for details. -
Q: What are the applications of MMP inhibitors?
A: MMP inhibitors are critical for studying disease states related to excessive ECM degradation, such as cancer metastasis, arthritis, and fibrosis. They can be used to evaluate the effects of inhibiting MMP activity in various biological processes.

