Protein Kinases
-
Gene
-
Species
-
Source
| Cat.No. | Product Name | Source | Species | Tag |
|---|
Background
Overview
Kinases are a class of molecules in biochemistry that transfer phosphate groups from high-energy donor molecules (such as ATP) to specific target molecules (substrates), a process called phosphorylation.
At present, the common kinase products in the industry are roughly divided into four categories: protein kinases, fat kinases, fructokinases and mutant kinases. The largest family of kinases are protein kinases.
Protein kinases act on specific proteins and alter their activity. These kinases play a wide range of roles in cellular signaling and its complex life activities. Different other kinases act on small molecules (lipids, sugars, amino acids, nucleosides, etc.), either to signal or to prepare them for various biochemical reactions in metabolism.
Differences Between Protein Kinases and Other Kinases
The main difference between protein kinases and other kinases is their substrate specificity and dependence.
Substrate specificity: Protein kinases act primarily on protein substrates, transferring phosphate groups to specific amino acid residues of proteins, such as serine, threonine, or tyrosine. While other types of kinases, such as lipokinases, act on fat molecules, fructokinases act specifically on fructose molecules.
Dependence: Some protein kinases are cyclic nucleotide dependent, such as cyclic adenylate (cAMP) protein kinases and cyclic guanosine (cGMP) protein kinases. These kinases play a key role in signaling by regulating activity through changes in the concentration of cyclic nucleotides. Other kinases may not rely on these second messenger molecules.
Functional diversity: The function of protein kinases is very diverse, they are involved in regulating a variety of intracellular signaling pathways, affecting cell growth, differentiation, metabolism and other physiological processes. Other types of kinases may play a role in more specific biochemical processes.
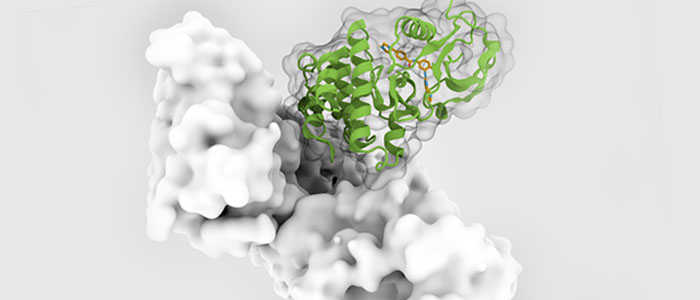
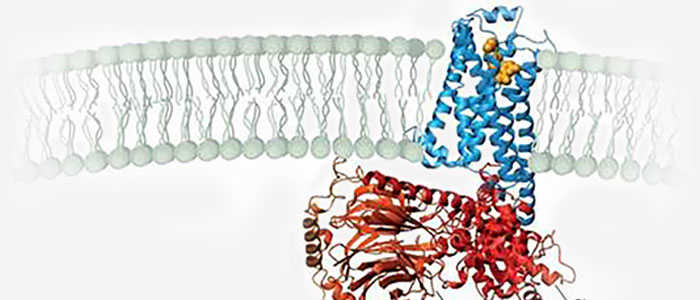
Structural characteristics: The structure of protein kinases usually contains a highly conserved catalytic core, which is responsible for ATP binding and phosphate transfer reactions. Other kinases may be structurally different to suit their specific substrate and reaction needs.
Functions
Protein kinases are a key family of enzymes, of which 518 members comprising more than 2% of the genome are involved in various cell signaling pathways in humans. Kinases exert their effects by phosphorylating their respective downstream specific substrate proteins. They catalyze the transport of γ-phosphate from ATP or GTP to hydroxyl groups of serine, threonine and tyrosine in substrate proteins or to glycosyl moieties in lipids. Kinase dysregulation can lead to changes in enzyme activity and/or enzyme expression, subcellular localization, kinase stability, protein-protein interactions, and the like. Dysregulation of kinase-mediated signaling pathways is responsible for all major human pathological conditions, such as cancer, cardiovascular disease, neurodegenerative diseases and metabolic disorders.
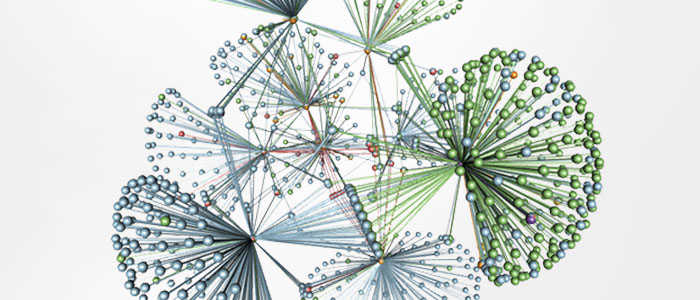
Members of the protein kinase superfamily are key regulators of cell signaling pathways. Kinases are involved in mediating a variety of important functions, as reflected in 50 distinct kinase families, some of which are conserved across yeast, invertebrates, and mammals. Of the 518 human protein kinases, 478 belong to a single superfamily whose catalytic domains are sequence-related. They can cluster into groups, families and subfamilies with increasing sequence similarity and biochemical function. The kinase dendrogram shows the sequence similarity between these catalytic regions: the distance along the branch between two kinases is proportional to the difference between the two sequences.
Genetic alterations of protein kinases impair their biological functions, resulting in dysregulation of cellular pathways such as apoptosis, cell cycle regulation, proliferation, angiogenesis, differentiation, and cellular metabolism. Mutations in kinase-encoding genes have been observed in many pathological conditions ranging from cancer, inflammatory disease, and cardiovascular disease to neurodegeneration. In addition, certain kinase mutations are associated with acquired drug resistance. Therefore, mutant kinases quickly become important drug targets, and become the focus of drug discovery and development efforts.
Categories
Protein kinases are classified mainly based on the type of amino acid residues they phosphorylate, and can be divided into the following five categories:
Serine/threonine protein kinases (ST-PKs): These kinases are the most common, and they phosphorylate serine or threonine residues in proteins.
Tyrosine protein kinases (TKs): These kinases specifically phosphorylate tyrosine residues, including some members of the TK family and TKL family.
Histidine specific kinases: These kinases specifically phosphorylate histidine residues.
Doubly specific protein kinase: Capable of phosphorylating serine/threonine and tyrosine residues.
Aspartate/glutamate specific protein kinases: These kinases target aspartate or glutamate residues.
In addition to the above categories, protein kinases can also be classified according to their activation conditions, such as:
Protein kinase A, also known as cyclic adenylate dependent protein kinase, is activated by a second messenger called cyclic adenylate and is found primarily in the cerebral cortex and striatum.
Protein kinase C: can be activated by diacylglycerol (DG).
Calmodulin protein kinase: can be activated by calcium ions and calmodulin.
Applications
The application of protein kinases covers many aspects from basic scientific research to clinical treatment, especially in the field of cancer treatment, kinase inhibitors have made remarkable progress. With a better understanding of kinase function and the development of new technologies, more innovative treatments are likely to emerge in the future. The application of protein kinases is mainly in the following areas:
- Medical research: Protein kinases play a crucial role in the intracellular transmission of nerve information by catalyzing the phosphorylation process of proteins and affecting the state change of ion channel proteins. This mechanism is particularly critical in the function of nerve cells, so protein kinases are of great value in the study of nervous system diseases.

- Drug development: The development of protein kinases as drug targets is one of the hot spots in modern pharmaceutical research. For example, small molecule kinase inhibitors inhibit protein kinase activity by binding to ATP binding sites, which has become the mechanism of action for many drugs. Most of the small molecule inhibitors approved by the FDA use this mechanism.

- Clinical treatment: Protein kinases play a role in cell growth, death, and the production of inflammatory mediators, so therapeutic strategies targeting kinases have shown efficacy in anticancer therapies and in the treatment of immune-mediated diseases. For example, certain kinase inhibitors are able to penetrate the blood-brain barrier and are used to treat brain metastases and pia meningeal diseases, which are difficult to achieve with traditional chemotherapy and radiotherapy.

Case Study
Case Study 1: Recombinant Human AAK1 Protein
This study attempts to develop and identify an aerobic glycolysis and glutamine-related genes (AGGRGs) signature for estimating prognostic effectively of ovarian cancer (OV) patients. OV related data were extracted from the multiple public databases, including TCGA-OV, GSE26193, GSE63885, and ICGC-OV. A consistent clustering approach was used to characterize the subtypes associated with AGGRGs. LASSO Cox regressions was utilized to construct the prognosis signatures of AGGRGs. In addition, GSE26193, GSE63885 and ICGC-OV served as independent external cohorts to assess the reliability of the model. In vitro and in vivo experiments were conducted to study the role of AAK1 in the malignant progression and glutamine metabolism of OV, and assessed its therapeutic potential for treating OV patients.
The team identified eight AGGRGs (AAK1, GJB6, HMGN5, LPIN3, INTS6L, PPOX, SPAG4, and ZNF316) to establish a prognostic signature for OV patients. In cytological experiments, we found that AAK1 could promote cancer progression and glutamine metabolism via activating the Notch3 pathway in OV cells. Furthermore, knockdown of AAK1 significantly inhibited tumor growth and weight, decreased lung metastases, and ultimately extended the survival time of the nude mice.
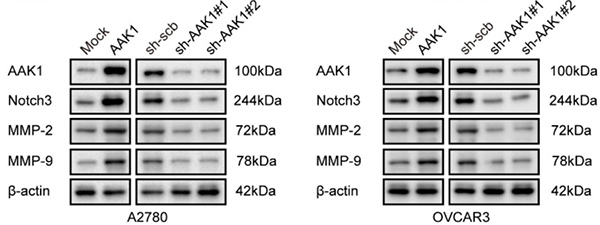
(Zihui Zhang, 2024)
Fig1. Western blotting showing the protein levels of AAK1, Notch3, MMP-2, and MMP-9 in A2780 and OVCAR3 cells stably transfected as indicated, respectively.
Case Study 2: Recombinant Human AATK Protein
Silencing of the Apoptosis associated Tyrosine Kinase gene (AATK) has been described in cancer. In this study, the researchers specifically investigated the epigenetic inactivation of AATK in pancreatic adenocarcinoma, lower grade glioma, lung, breast, head, and neck cancer. The resulting loss of AATK correlates with impaired patient survival. Inhibition of DNA methyltransferases (DNMTs) reactivated AATK in glioblastoma and pancreatic cancer.
In contrast, epigenetic targeting via the CRISPR/dCas9 system with either EZH2 or DNMT3A inhibited the expression of AATK. Via large-scale kinomic profiling and kinase assays, we demonstrate that AATK acts a Ser/Thr kinase that phosphorylates TP53 at Ser366. Furthermore, whole transcriptome analyses and mass spectrometry associate AATK expression with the GO term 'regulation of cell proliferation'. The kinase activity of AATK in comparison to the kinase-dead mutant mediates a decreased expression of the key cell cycle regulators Cyclin D1 and WEE1. Moreover, growth suppression through AATK relies on its kinase activity.
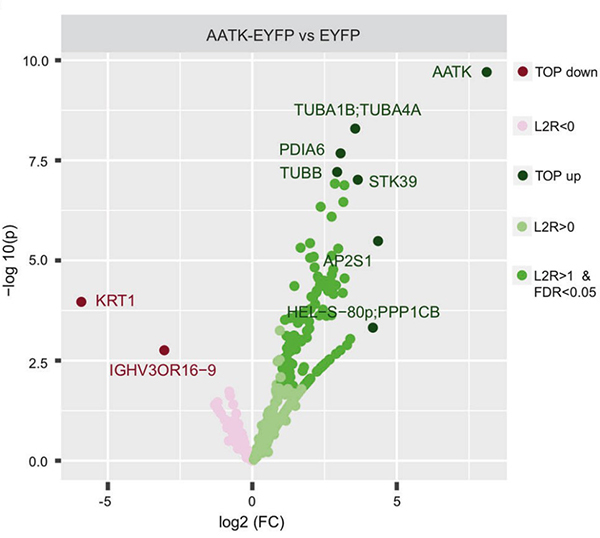
(Michelle L Woods, 2022)
Fig2. Binding partners of AATK were detected in HEK293T cells after transfection of EYFP-tagged AATK and EYFP empty and subsequent pulldown with GFP-Trap and mass spectrometry.
Case Study 3: Recombinant Human ADCK1 Protein
As a result of mutations in the upstream components of the Wnt/β-catenin signaling pathway, this cascade is abnormally activated in colon cancer. Hence, identifying the activation mechanism of this pathway is an urgent need for the treatment of colon cancer. Here, the researchers found an increase in ADCK1 (AarF domain-containing kinase 1) expression in clinical specimens of colon cancer and animal models. Upregulation of ADCK1 expression promoted the colony formation and infiltration of cancer cells. Downregulation of ADCK1 expression inhibited the colony formation and infiltration of cancer cells, in vivo tumorigenesis, migration, and organoid formation. Molecular mechanistic studies demonstrated that ADCK1 interacted with TCF4 (T-cell factor 4) to activate the β-catenin/TCF signaling pathway.
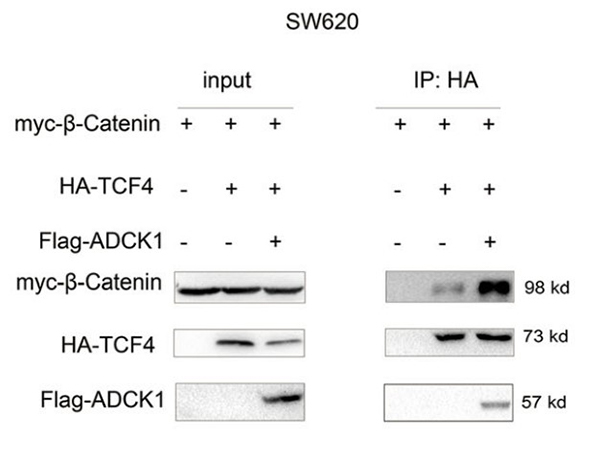
(Yong Ji, 2021)
Fig3. Co-IP was performed to examine the effects of ADCK1 overexpression on the interaction between β-catenin and TCF4 in SW620 cells.
Case Study 4: Recombinant Human AKT1 Protein
Overexpression or activation of AKT is very well known to control cell growth, survival, and gene expression in solid tumors. In this study, we demonstrated that oridonin is an inhibitor of AKT and suppresses proliferation of esophageal squamous cell carcinoma (ESCC) in vitro and in vivo The role of AKT in ESCC was studied using immuno-histochemical analysis of a tumor microarray, the effect of AKT knockdown on cell growth, and treatment of cells with MK-2206, an AKT inhibitor. Oridonin blocked AKT kinase activity and interacted with the ATP-binding pocket of AKT.It inhibited growth of KYSE70, KYSE410, and KYSE450 esophageal cancer cells in a time- and concentration-dependent manner. Oridonin induced arrest of cells in the G2-M cell-cycle phase, stimulated apoptosis, and increased expression of apoptotic biomarkers, including cleaved PARP, caspase-3, caspase-7, and Bims in ESCC cell lines. Mechanistically, we found that oridonin diminished the phosphorylation and activation of AKT signaling. Furthermore, a combination of oridonin and 5-fluorouracil or cisplatin (clinical chemotherapeutic agents) enhanced the inhibition of ESCC cell growth.
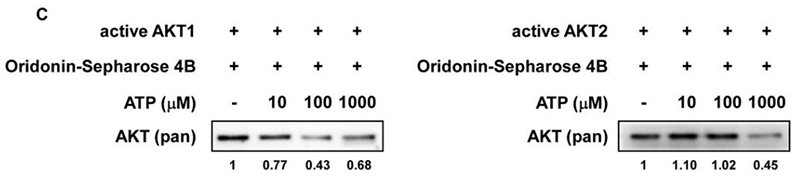
(Mengqiu Song, 2018)
Fig4. The specificity of the binding of oridonin to active AKT1 or AKT2 in the presence of ATP was evaluated.
Advantages
- Strong R&D capability: We have strong R&D team and advanced technology platform can continuously develop and optimize protein kinase products to maintain technology leadership.
- Good product quality: The protein kinase products we provided have the characteristics of high purity, stable activity, etc., to meet the needs of different research and applications.
- Product diversity: Our product lines are rich, covering multiple types of protein kinases, suitable for multiple research areas and application scenarios.
- Customized services: We have the ability to provide customized protein kinase products to meet the needs of specific research or industrial applications.
- Technical support: We can provide professional technical support and after-sales service to help our customers solve problems during use.
FAQ
-
Q: What are the sources of your protein kinase products?
A: Our range of products covers common animal species such as humans, mice, chickens and monkeys, as well as other microorganisms. If you have special needs, you can also contact us for customized services.
-
Q: How about the quality of the product? Are there any tests available?
A: We ensure the high quality and accuracy of our products through a variety of detection techniques, including SDS-PAGE and WB, as well as isotope labeling analysis, mass spectrometry and more.
References
- Zhang Z.; et al. Comprehensive analysis based on glycolytic and glutaminolytic pathways signature for predicting prognosis and immunotherapy in ovarian cancer. J Cancer. 2024;15(2):383-400.
- Zhu Q.; et al. Down-regulation of PBK inhibits proliferation of human endometrial stromal cells in thin endometrium. Reprod Biol Endocrinol. 2022;20(1):25.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions