Type I Interferon Signal Pathway

🧪 EIF4E-2775H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-217 aa
🧪 EIF4EBP1-1397H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-118 a.a.

🧪 RAC1-2213H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: Met1-Cys189
🧪 Ifnar1-3360M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: 1-429 a.a.
🧪 IFNAR2-3349H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 1-243 a.a.
🧪 STAT1-2783H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-712 a.a.
🧪 EIF4EBP1-3804H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: Ser2-Ile118
🧪 MTOR-30254TH
Source: Wheat Germ
Species: Human
Tag: Non
Conjugation:
Protein Length: 100 amino acids
🧪 STAT1-7037H
Source: Insect Cells
Species: Human
Tag:
Conjugation:
Protein Length: Met1-Val712
🧪 STAT2-7056H
Source: Sf9 Cells
Species: Human
Tag:
Conjugation:
Protein Length: Full length
🧪 IFNAR2-14078H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 230-331a.a.
🧪 RAC1-2147H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-192aa
🧪 RPS6KA5-2435H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-319aa
🧪 RPS6KB2-2437H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-325aa
🧪 STAT1-2996H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 2-230 aa
Background of VEGF Signaling Pathway
What is VEGF Signaling Pathway?
VEGF (Vascular Endothelial Growth Factor) signaling pathway is a key signaling network in cell biology, and it plays a crucial role in angiogenesis. Angiogenesis is the formation of new blood vessels from existing vascular systems, a process that is important for a variety of physiological and pathological conditions, including development, wound healing, the female menstrual cycle, pregnancy, and tumor growth.
The core of VEGF signaling pathway is the VEGF protein family, which includes A variety of isoforms, such as VEGF-A, VEGF-B, VEGF-C, VEGF-D, etc. They work by binding to the corresponding Receptor, VEGFR (Vascular Endothelial Growth Factor Receptor). The VEGFR family includes VEGFR1, VEGFR2 and VEGFR3, of which VEGFR2 is the main receptor mediating angiogenesis.
VEGF binding to the extracellular portion of VEGF R2 leads to receptor dimerization, activation of its intracellular tyrosine kinase domains, and autophosphorylation. Major sites of human VEGF R2 phosphorylation have been characterized and include Tyr801, Tyr951, Tyr1054, Tyr1059, Tyr1175, and Tyr1214. Additional phosphorylation sites on VEGF R2 include Tyr1223, Tyr1305, Tyr1309, and Tyr1319, however the function of phosphorylation at these sites is not well-defined. Autophosphorylation of VEGF R2 on Tyr1054 and Tyr1059 upon receptor dimerization is critical for downstream kinase activity and may be preceded by autophosphorylation on Tyr801. The other major phosphorylation sites on VEGF R2 are necessary for the recruitment of downstream signaling molecules such as Src, SHB, PI 3-K, NCK/Fyn, CDC42, PLC-gamma, GRB2/SOS/Shc, and SH2D2A. Interaction sites on VEGF R2 for the recruitment of GRB2/GAB1, C3G/Crk, and Jak have not been identified.
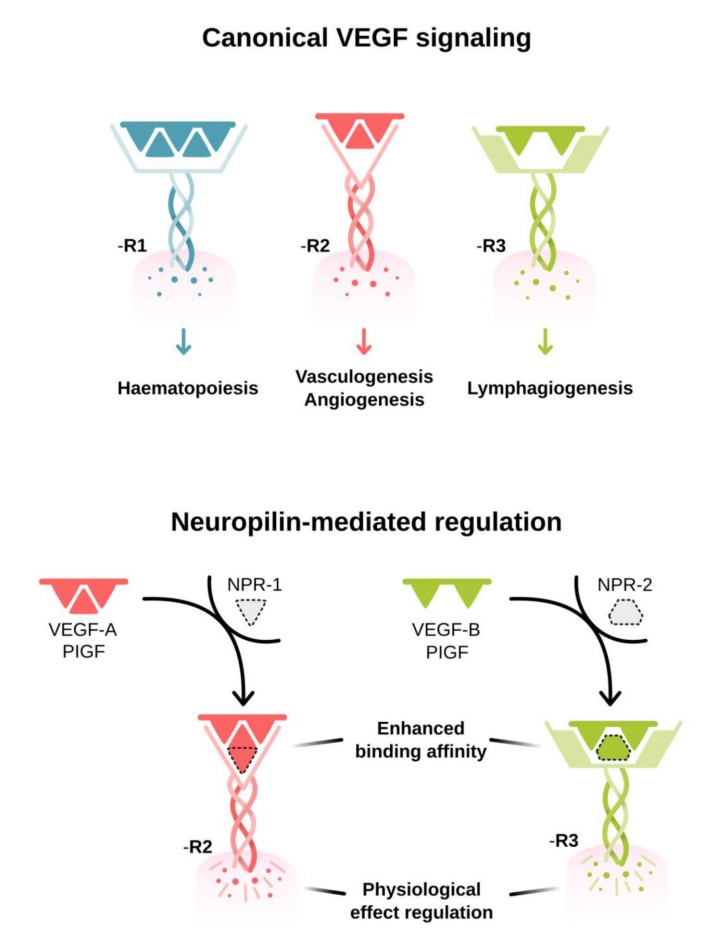
Fig1. VEGF activation and signaling pathways. (Rajendra S Apte, 2019)
Downstream Signaling Cascades
Common downstream signaling cascades initiated by VEGF R2 recruitment of these signaling molecules include the ERK1/2 MAP Kinase, FAK, p38 MAP Kinase, Akt, and Jak-STAT pathways, which result in the activation of a full range of biological responses that regulate angiogenesis including endothelial cell proliferation, survival, adhesion, and migration, as well as vascular permeability.
Ras/RAF/MEK/ERK/MAPK pathway: After VEGF binds to VEGFR, Ras protein can be activated, which in turn activates RAF, leading to the phosphorylation of MEK and ERK, and ultimately promotes cell proliferation and gene expression.
PI3K/AKT pathway: VEGF-VEGFR binding also activates phosphatidylinositol-3-kinase (PI3K), which in turn activates AKT (protein kinase B). AKT is involved in regulating a variety of biological processes, including cell survival, proliferation, metabolism, and cell cycle processes.
JAK/STAT pathway: VEGF signaling also activates Janus kinase (JAK), leading to phosphorylation and activation of signal transduction and activator of transcription (STAT), which then enters the nucleus and activates target genes.
PLCγ/Ca2+ pathway: The activation of VEGFR can lead to the activation of phospholipase Cγ (PLCγ), which produces inositol triphosphate (IP3) and diacylglycerol (DAG) by hydrolyzing phosphatidylinositol 4, 5-diphosphate (PIP2), which cause an increase in intracellular calcium concentration and activation of protein kinase C (PKC), respectively.
FAK/paxillin pathway: VEGF signaling is also involved in proteins such as adhesion spot kinase (FAK) and paxillin, which are involved in cytoskeleton rearrangement and cell migration.
Roles of VEGF Signaling Pathway
Development and Growth: VEGF is essential for embryonic development, particularly in the formation of the vascular system. It guides the growth and differentiation of blood vessels during organogenesis.
Wound Healing: After injury, VEGF is involved in the healing process by stimulating the growth of new blood vessels to the damaged area, which is critical for tissue repair and regeneration.
Tumor Angiogenesis: In cancer, VEGF signaling is often hijacked by tumors to stimulate the formation of new blood vessels that supply them with oxygen and nutrients, thereby promoting their growth and metastasis.
Inflammation and Immune Response: VEGF is involved in the inflammatory process by promoting the permeability of blood vessels, allowing the passage of immune cells to the site of inflammation.
Cardiovascular Health: VEGF influences cardiovascular health by regulating the growth and maintenance of blood vessels, which can impact conditions such as atherosclerosis and hypertension.
Diagnosis and Prognosis: Measuring VEGF levels can be diagnostically useful in determining the presence and aggressiveness of diseases, as well as providing prognostic information regarding treatment outcomes.
Pathway Related Diseases
Cancer: VEGF is a key driver of tumor angiogenesis, which is the formation of new blood vessels that supply tumors with the oxygen and nutrients they need to grow. High levels of VEGF are found in many types of cancer, including glioblastoma, non-small cell lung cancer, renal cell carcinoma, and colorectal cancer. Targeting the VEGF pathway can inhibit this process and is a strategy used in several anti-cancer therapies.
Age-Related Macular Degeneration (AMD): VEGF is involved in the abnormal growth of blood vessels in the retina, which can lead to vision loss in conditions like AMD. Anti-VEGF therapies are used to treat this disease by inhibiting the growth of these vessels.
Diabetic Retinopathy: Similar to AMD, in diabetic retinopathy, VEGF contributes to the growth of abnormal blood vessels in the retina, which can cause vision impairment. Anti-VEGF treatments are also used in this condition.
Preeclampsia: This pregnancy complication is characterized by high blood pressure and damage to organs, including the liver and kidneys. VEGF is thought to play a role in the development of preeclampsia by affecting vascular function.
Neurological Conditions: VEGF has been implicated in several neurological conditions, including epilepsy, brain tumors, stroke, and neuromyelitis optica. It is thought to play a role in neuronal protection and the repair of brain tissue after injury.
Wound Healing and Regeneration: VEGF is important for normal wound healing and tissue regeneration. However, in some chronic wounds, such as those seen in diabetes, the balance of VEGF signaling may be disrupted, leading to impaired healing.
Cardiovascular Diseases: VEGF is involved in the formation of new blood vessels following tissue ischemia, which can be a critical process in the healing of heart tissue after a heart attack. However, dysregulation of VEGF signaling can also contribute to certain cardiovascular diseases.
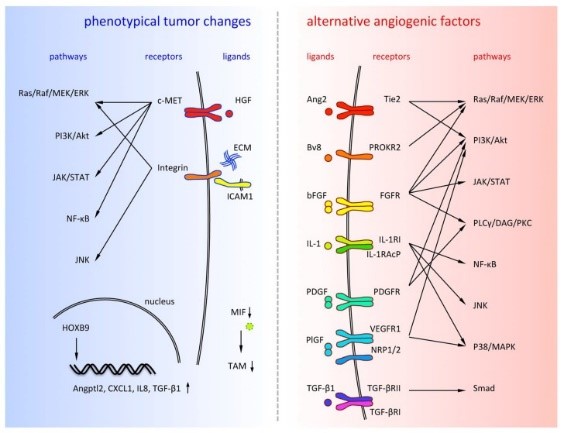
Fig2. Alternative angiogenic factors are listed on the right side and phenotypical tumor changes are listed on the left side. (Yoshiro Itatani, 2018)
The VEGF pathway is a significant therapeutic target, and drugs that inhibit VEGF or its receptors have been developed to treat various diseases, particularly cancers and eye diseases. These drugs work by blocking the signals that promote the growth of new blood vessels, thereby limiting the supply of nutrients to the diseased tissues.
Case Study
Case Study 1: Recombinant Human PRKDC Protein (PRKDC-860H)
Small cell lung cancer (SCLC) is a type of neuroendocrine tumor characterized by rapid spread and resistance to chemotherapy drugs. The genomic instability of this tumor is due in part to high mutation rates, frequent deletion of TP53 and RB1 genes, and specific amplification patterns of members of the MYC gene family. These factors complicate the development of targeted treatments. Previously, researchers identified a novel OCT4-driven transcriptional activation mechanism of MYC gene in neuroblastoma, another neuroendocrine tumor, involving c-MYC, pOCT4S111, and MAPKAPK2. Now, by performing tumor microarray analysis on clinical samples, combined with pre-clinical model studies, the researchers have observed correlations in the expression of these proteins in SCLC. Further experiments confirmed the direct interaction between OCT4 and DNA-PKcs and revealed their specific binding sites. By decreasing the expression of POU5F1 and PRKDC (encoding OCT4 and DNA-PKcs, respectively), a reduction in c-MYC expression was observed.
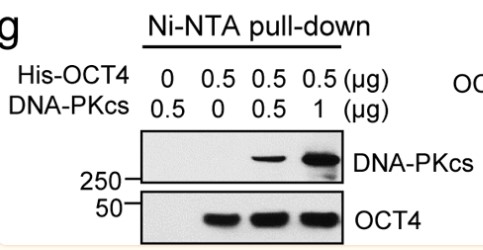
Fig1.OCT4 binds to DNA-PKcs in a cell-free system. (Sung-Jen Wei, 2023)
Case Study 2: Recombinant Human CREB1 (CREB1-26H)
This study was aimed to establish graded NP model to investigate the effect of CREB1 on nerve repair and neuropathic pain (NP) after peripheral nerve injury. Luxol fast blue staining was performed to measure the ratio of distal myelin sheath to proximal (DPR). The c-Fos, GFAP, CX3CR1 and IBA-1 expressions in spinal cord were measured by Western blot. The expression levels of CREB1 and ATF-3 in dorsal root ganglion (DRG) were both measured. Intrathecal injection was performed by using recombinant CREB, or anti-CREB antibody for NP model, respectively. The above indexes were detected. The CREB1 and ATF-3 expressions in DRG showed gradually increase. With the injection of recombinant CREB, the similar changes were found in rats compared with NP model. While after anti-CREB1 antibody injection, all effects of CREB1 were impaired. Likewise, anti-CREB1 antibody treatment increased 50% PWT and DPR, decreased SFI, decreased expressions of c-Fos, GFAP, CX3CR1 and IBA-1. Besides, ATF-3 expression was inhibited by CREB1 suppression.
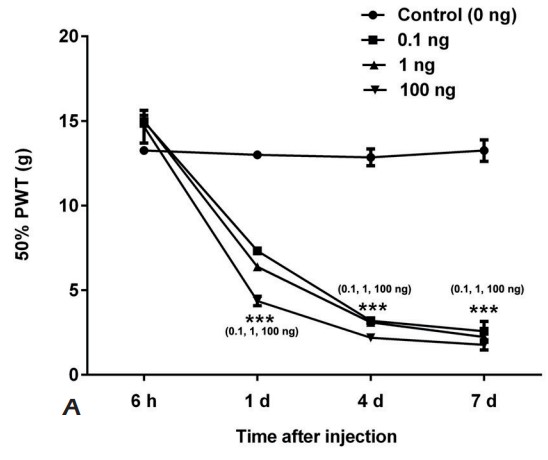
Fig2. The 50% PWT of SD rats injected with 0, 0.1, 1, or 100 ng recombined CREB1 was measured. (T Tao, 2019)
Case Study 3: Recombinant Human SRC protein (SRC-533H)
Tricellular junctions (TCJs) are uniquely placed permeability barriers formed at the corners of polarized epithelia where tight junctions in vertebrates or septate junctions (SJ) in invertebrates from three cells converge. Gliotactin is a Drosophila TCJ protein, and loss of Gliotactin results in SJ and TCJ breakdown and permeability barrier loss. When overexpressed, Gliotactin spreads away from the TCJs, resulting in disrupted epithelial architecture, including overproliferation, cell delamination, and migration. Gliotactin levels are tightly controlled at the mRNA level and at the protein level through endocytosis and degradation triggered by tyrosine phosphorylation. The researchers identified C-terminal Src kinase (Csk) as a tyrosine kinase responsible for regulating Gliotactin endocytosis. Increased Csk suppresses the Gliotactin overexpression phenotypes by increasing endocytosis. Loss of Csk causes Gliotactin to spread away from the TCJ. Although Csk is known as a negative regulator of Src kinases, the effects of Csk on Gliotactin are independent of Src and likely occur through an adherens junction associated complex.
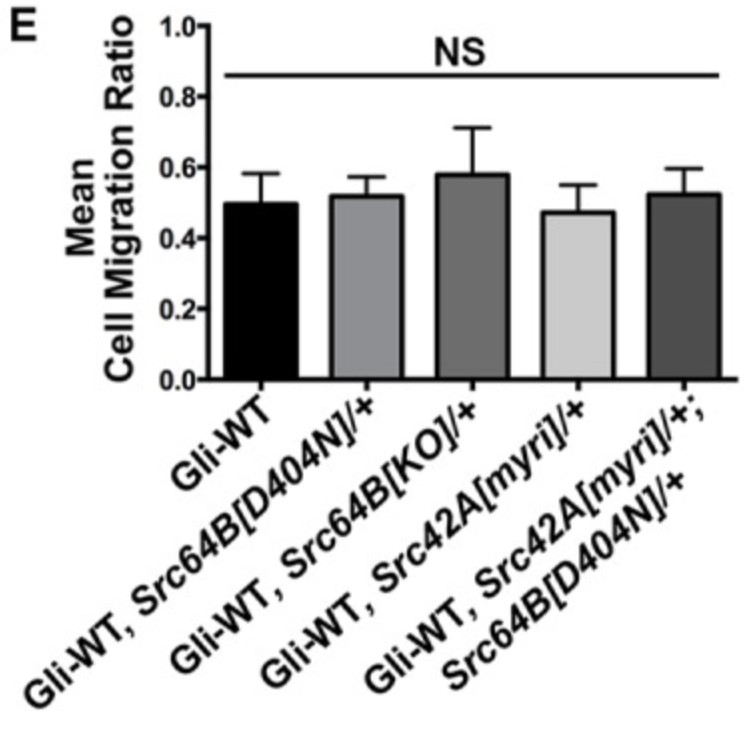
Fig3. Statistical analysis of cell migration ratios in Gliotactin overexpression alone (ap>Gli-WT) compared with expression in Src heterozygous mutant backgrounds. (G D N Gayathri Samarasekera, 2018)
Related Products
VEGF signaling pathway plays a crucial role in a variety of physiological and pathological processes, primarily through its regulation of angiogenesis, which is the formation of new blood vessels from pre-existing ones. Focusing on the life sciences sector, Creative BioMart offers a range of high-quality products related to the VEGF signaling pathway. Please contact us if you have any need.


