Biomarker
-
Gene
-
Species
-
Source
| Cat.No. | Product Name | Source | Species | Tag |
|---|
Background
Overview
A biomarker is a substance that is measured in a biological system as an indicator of exposure, effect, susceptibility, or clinical disease. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.
WHO has defined a biomarker as “any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease”. An even broader definition takes into account not just the incidence and outcome of disease, but also the effects of treatments, interventions, and even unintended environmental exposure, such as to chemicals or nutrients.

Features
The definition of a biomarker involves several key elements:
Measurable: Biomarkers must be objectively measurable by some method, meaning they can be detected in a laboratory or clinical setting.
Reflecting physiological or pathological processes: Biomarkers can provide information about an individual's health status, including the onset, progression, and effect of treatment.
Biological effects: Biomarkers can also be used to assess the effects of environmental exposures, drugs or other interventions on the human body.
Materiality and metrology: Biomarkers are usually derived from human tissues or body fluids, which are biologically materialistic and have metrology associated with human physiological and pathological processes.
Categories
Biomarkers are classified mainly based on their function and can be divided into the following six categories:
Diagnostic biomarkers: Biomarkers used to detect or confirm disease status, or to identify disease subtypes. For example, certain tumor markers can help doctors determine if a patient has a specific type of cancer.
Prognostic biomarkers: These biomarkers are used to predict the progression of the disease, such as whether the disease will worsen or how well the patient will respond to treatment.
Predictive biomarkers: Used to predict the development of a disease, for example, certain genetic variants that may predict a patient's response to a particular drug.
Pharmacodynamic biomarkers: These markers are used to assess the efficacy of a drug, that is, whether the drug treatment has achieved its intended effect.
Safety biomarkers: Used to monitor the safety of a drug or treatment to detect potential adverse reactions or side effects in a timely manner.
Monitoring biomarkers: used to monitor the therapeutic effect and prognosis of diseases, such as tumor markers and inflammatory markers.
In addition, according to the nature, biomarkers can also be divided into proteins, nucleic acids, metabolites and so on. For example, C-reactive protein in the blood, liver function indicators such as ALT and AST, and tumor markers such as CEA and AFP are all protein biomarkers.

Differences Between Different Types of Biomarkers
The difference between different types of biomarkers is mainly reflected in their function and application. The specific analysis is as follows:
Functional differences:
Diagnostic biomarkers are used to detect or confirm disease status and help doctors determine the type and presence of disease.
Prognostic biomarkers can predict the progression of a disease, such as whether it will get worse.
Predictive biomarkers are primarily used to predict how a disease will respond to treatment, as well as the patient's likely clinical outcome.
Pharmacodynamic biomarkers are used to evaluate the efficacy of a drug and determine whether a drug treatment is effective.
Safety biomarkers are used to monitor the safety of a drug or treatment to detect potential adverse reactions or side effects in a timely manner.
Monitoring biomarkers are used to track the therapeutic effect and prognosis of diseases, such as tumor markers and inflammatory markers.
Application differences:
Diagnostic biomarkers are especially important in the early diagnosis of disease and can help doctors take timely treatment measures.
Prognostic and predictive biomarkers have guiding significance for the development of personalized treatment and evaluation of treatment effect.
Efficacy and safety biomarkers are critical in new drug development and clinical trials to assess the potential and risks of new drugs.
Monitoring biomarkers are very useful in chronic disease management and can help doctors and patients understand changes in the condition.
Research Progress
Advances in biomarker research are involved in several areas, including the field of disease and detection:
Disease:
Early screening and diagnosis of breast cancer is the key to reduce mortality and improve prognosis. Currently, mammography screening, while widely used, has problems with false positives, radiation, and overdiagnosis. Therefore, researchers are developing easy-to-access, stable and reliable biomarkers for non-invasive screening and diagnosis of breast cancer.
Acute kidney injury is a serious medical condition and prompt diagnosis is critical. Researchers are exploring new biomarkers to identify and treat acute kidney injury earlier.
Detection:
Immunology-based assays (e.g., antigens/antibodies) are easy to use, portable and suitable for rapid detection.
The combined use of microchip technology provides new possibilities for the detection of biomarkers, making multiple detection more efficient and accurate.

Applications
The application of biomarkers is very wide, involving early diagnosis of diseases, treatment prediction, clinical staging and efficacy monitoring. Here are some specific application examples:
- Early diagnosis: Biomarkers can help doctors diagnose the disease at an early stage, which is crucial for the treatment and prognosis of many diseases.
- Risk stratification: By detecting specific biomarkers, patients can be divided into different risk groups, allowing for more personalized treatment options for patients.
- Prediction of efficacy: Certain biomarkers are able to predict a patient's response to a particular treatment, which helps doctors choose the most effective treatment.
- Clinical staging: In the field of oncology, biomarkers can help physicians determine the stage of disease development, which is important for developing treatment plans and assessing prognosis.
- Monitoring efficacy: During treatment, by monitoring changes in biomarkers, the therapeutic effect can be evaluated and the treatment regimen can be adjusted.
- Research and development applications: In drug development, biomarkers can be used as inclusion/exclusion criteria, or as efficacy endpoints/surrogate endpoints to guide dose selection.
- Omics application: The application of biomarkers can help researchers understand the specific biochemical indicators of diseases, and play a key role in the diagnosis and prevention of diseases.
In environmental science, biomarkers can also be used to clarify the mechanism of action of pollutants, determine the interaction between pollutants and organisms, and link to ecological effects to help develop solutions to environmental problems.
Case Study
Case Study 1: Recombinant Human ALPP Protein
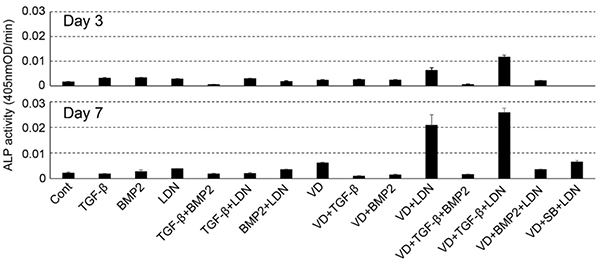
Human umbilical cord perivascular cells (HUCPVCs), harvested from human umbilical cord perivascular tissue, show potential for future use as an alternative to mesenchymal stromal cells. Here, the researchers present the results for the characterization of the properties alkaline phosphatase-positive HUCPVCs (ALP(+)-HUCPVCs). These ALP(+)-HUCPVCs were created from HUCPVCs in this study by culturing in the presence of activated vitamin D3, an inhibitor of bone morphogenetic protein signaling and transforming growth factor-beta1 (TGF-β1). The morphological characteristics, cell proliferation, gene expression, and mineralization-inducing ability of ALP(+)-HUCPVCs were investigated at the morphological, biological, and genetic levels. ALP(+)-HUCPVCs possess high ALP gene expression and activity in cells and a slow rate of cell growth. The morphology of ALP(+)-HUCPVCs is fibroblast-like, with an increase in actin filaments containing alpha-smooth muscle actin. In addition to ALP expression, the gene expression levels of type I collagen, osteopontin, elastin, fibrillin-1, and cluster of differentiation 90 are increased in ALP(+)-HUCPVCs. ALP(+)-HUCPVCs do not have the ability to induce mineralization nodules, which may be due to the restriction of phosphate uptake into matrix vesicles. Moreover, ALP(+)-HUCPVCs may produce anti-mineralization substances.
(Shun Nonoyama, 2021)
Fig1. Effect of various factors on the ALP-inducing activity of HUCPVCs.
Case Study 2: Recombinant Human ANGPT2 Protein
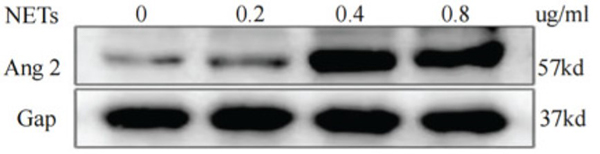
Although significant progress has been made in the study of gastric cancer (GC), clinicians lack reliable protein markers for accurate diagnosis and tumor stratification. Neutrophil extracellular traps (NETs) are networks of extracellular fibers composed of DNA from neutrophils. The researchers have previously reported that abundant NETs are deposited in GC, damaging human umbilical vein endothelial cells (HUVECs) and triggering the release of tissue factors, leading to a hypercoagulable state in GC. However, the specific effects of NETs on HUVECs are unclear. This study aimed to explore the functional changes caused by NETs on HUVECs, providing evidence that NETs may fuel GC progression. Through quantitative proteomics, they identified 6182 differentially expressed proteins in NET-stimulated HUVECs by TMT. The reliability of the TMT technique was confirmed by parallel reaction monitoring (PRM) analysis of 17 differentially expressed proteins. Through bioinformatics analysis, we found that NETs upregulate ANGPT2 in HUVECs. They comprehensively analyzed the prognosis, biological function, immune response, and therapeutic value of ANGPT2 in GC. They found that overexpression of ANGPT2 in GC is associated with poor prognosis and potentially regulates multiple biological functions. At the same time, ANGPT2 also predicted immunotherapeutic and chemotherapeutic responses in GC.
(Shifeng Yang, 2022)
Fig2. Western blot analysis of ANGPT2 expression after HUVECs were stimulated by different concentrations of NETs.
Case Study 3: Recombinant Rat B2M Protein
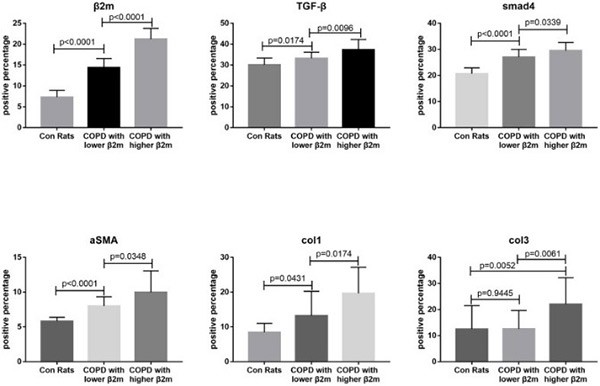
Expression of β2-microglobulin (β2M) is involved in fibrosis progression in kidney, liver, and heart. In this case-controlled retrospective study, the researchers investigated the role of β2M in the development of pulmonary fibrosis in patients with chronic obstructive pulmonary disease (COPD). Analysis of 450 COPD patients revealed that patients with decreased pulmonary diffusing capacity (DLCO) had increased β2M serum levels. Compared to patients with lower β2M serum levels, patients with increased β2M levels exhibited increased alveolar wall/septal thickening and lung tissue β2M expression. In addition, patients with increased β2M levels had increased lung expression of TGF-β1, Smad4, and a-SMA. Animal experiments showed that increased β2M expression resulted in epithelial-mesenchymal transition (EMT), alveolar wall/septal thickening, and pulmonary fibrosis in a rat COPD model.
(Zhenchao Wu, 2020)
Fig3. Immunohistochemical staining quantitative traits of lung tissue from rats.
Case Study 4: Recombinant Human BCL2 Protein
Chimeric antigen receptor T-cell (CART) immunotherapy led to unprecedented responses in patients with refractory/relapsed B-cell non-Hodgkin lymphoma (NHL); nevertheless, two thirds of patients experience treatment failure. Resistance to apoptosis is a key feature of cancer cells, and it is associated with treatment failure. In 87 patients with NHL treated with anti-CD19 CART, the researchers found that chromosomal alteration of B-cell lymphoma 2 (BCL-2), a critical antiapoptotic regulator, in lymphoma cells was associated with reduced survival. Therefore, they combined CART19 with the FDA-approved BCL-2 inhibitor venetoclax and demonstrated in vivo synergy in venetoclax-sensitive NHL. However, higher venetoclax doses needed for venetoclax-resistant lymphomas resulted in CART toxicity. To overcome this limitation, they developed venetoclax-resistant CART by overexpressing mutated BCL-2(F104L), which is not recognized by venetoclax. Notably, BCL-2(F104L)-CART19 synergized with venetoclax in multiple lymphoma xenograft models.
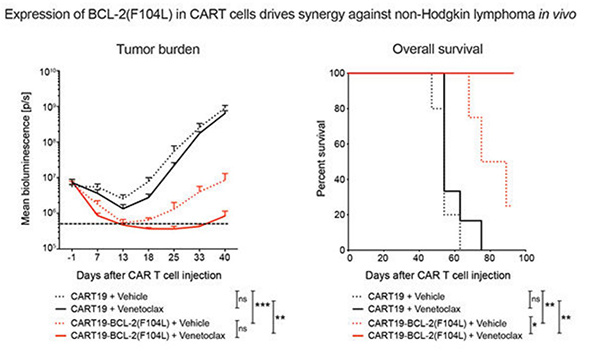
(Yong Gu Lee, 2022)
Fig4. Tumor progression and survival of xenografted mice bearing MINO. Treated with CART19 or CART19-BCL-2(F104L) plus either vehicle or venetoclax.
Advantages
- Strong R&D capability: We have strong R&D team and advanced technology platform can continuously develop and optimize protein kinase products to maintain technology leadership.
- Market recognition: We have a good market reputation and brand influence, products are widely recognized and used.
- Cooperation network: Extensive cooperation with academia, research institutions and industry has been established, which is conducive to the continuous innovation of technology and the promotion of applications.
- Customized services: We have the ability to provide customized biomarkers products to meet the needs of specific research or industrial applications.
References
- Nonoyama S.; et al. Development and Characterization of Alkaline Phosphatase-Positive Human Umbilical Cord Perivascular Cells. Cells. 2021;10(11):3011.
- Wu Z.; et al. β2-microglobulin as a biomarker of pulmonary fibrosis development in COPD patients. Aging (Albany NY). 2020;13(1):1251-1263.
- Lee YG.; et al. Modulation of BCL-2 in Both T Cells and Tumor Cells to Enhance Chimeric Antigen Receptor T-cell Immunotherapy against Cancer. Cancer Discov. 2022;12(10):2372-2391.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions