Downstream Processing Development
Background on Downstream Processing in Biologic Drug Development
Downstream processing refers to the critical recovery and purification stages in the production of biopharmaceuticals, occurring after upstream fermentation or cell culture. This phase is essential for isolating the target recombinant product—such as therapeutic proteins, antibodies, or vaccines—while removing impurities, host cell proteins, DNA, endotoxins, and other process-related contaminants. A successful downstream process must preserve the structural integrity, biological activity, and potency of the product, all while ensuring high yield and regulatory compliance.
DSP typically includes the following key steps:
- Cell Harvesting : Removes cells and debris from the culture broth using centrifugation or filtration.
- Primary Recovery / Clarification : Further clears the supernatant, often using depth filtration or microfiltration.
- Capture / Initial Purification : Concentrates and isolates the target molecule using techniques like affinity chromatography (e.g., Protein A for antibodies).
- Intermediate and Polishing Steps : Additional chromatography steps (e.g., ion exchange, hydrophobic interaction, size exclusion) remove impurities like host cell proteins, DNA, and aggregates.
- Viral Clearance : Ensures safety by removing or inactivating potential viral contaminants via filtration or chemical treatment.
- Formulation and Final Filtration : Buffers are exchanged, and stabilizers are added to create the final drug product, followed by sterile filtration and aseptic filling.
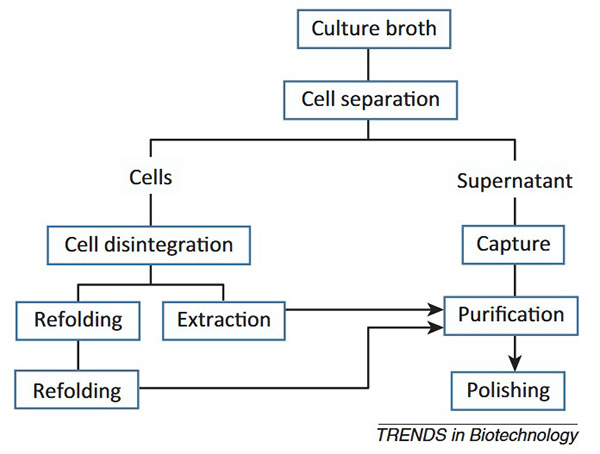
Figure 1. General flowchart of downstream processing of recombinant proteins irrespective of the starting material. (Jungbauer, 2013)
At Creative BioMart , we collaborate closely with clients to design, optimize, and validate downstream purification processes tailored to their specific biotherapeutic candidates. Our experienced team applies industry best practices and leverages a robust knowledge base to deliver scalable, cost-effective solutions that meet stringent quality, quantity, and compliance standards.
Our Downstream Process Development Services for Biopharmaceuticals
We support the entire spectrum of downstream development—from early-stage purification to large-scale manufacturing—and provide pre-clinical and GMP-grade material upon request.
Service Procedure

Service Details
Our downstream process development strategy is guided by a commitment to using commercially available, regulatory-friendly technologies that ensure smooth tech transfer to GMP production. Whether developing a novel process or improving an existing one, Creative BioMart provides full transparency, thorough documentation, and a deep understanding of the unique challenges associated with each product.
-
Downstream Purification Optimization
Tailored selection and optimization of chromatography and filtration strategies to achieve desired purity, yield, and scalability.
-

Development & Validation of Viral Inactivation Steps
Design and verification of robust viral inactivation/removal unit operations to ensure safety and compliance with regulatory guidelines.
-
Pilot-Scale and Production-Scale Process Development
Seamless scale-up from lab bench to pilot and full production volumes while maintaining product integrity and process reproducibility.
-

GMP-Compliant Process Validation
Full validation packages aligned with regulatory expectations, including consistency runs and impurity clearance verification.
-

Cost of Goods (CoG) Analysis
Economic modeling to assess the long-term feasibility of the selected downstream process in commercial manufacturing.
Core Techniques and Technologies
We deploy a wide range of advanced downstream techniques, each selected and customized based on the specific properties of your molecule:
|
Type |
Details |
|---|---|
|
Cell Disruption |
Sonication and high-pressure homogenization for efficient recovery of intracellular proteins. |
|
Chromatography Techniques |
|
|
Ultrafiltration/Diafiltration (UF/DF) |
For buffer exchange, concentration, and polishing. |
|
Membrane-Based Separation Technologies |
Customized selection of membrane types and configurations. |
|
Viral Clearance Studies |
Integration of orthogonal virus removal and inactivation steps, compliant with regulatory expectations. |
Advantages of Our Downstream Process Development Services
- Full-Service Partnership : From early development to clinical trial support, we serve as your reliable partner.
- Flexible Project Engagement Models : Customizable services for all stages and sizes of development projects.
- Scientific Expertise : Decades of experience across biologics and vaccines, including mAbs, fusion proteins, and enzymes.
- Advanced Technologies & Infrastructure : Automated systems and modern tools ensure fast, reliable purification processes.
- Transparent Communication : Clients receive detailed process development reports including method evaluation, justification of selected approaches, and clear documentation for regulatory filings.
- Scalability & Tech Transfer Readiness : Our downstream processes are designed with scale-up and manufacturing transfer in mind.
Case Studies: Strategies for Optimizing Downstream Processing
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Cost-effective downstream processing of algal biomass for industrial-scale biofuels production
Banerjee et al ., 2024. doi:10.1007/978-3-031-52319-9_11
The downstream processing of microalgae involves harvesting, drying, extraction, and purification of valuable metabolites. Harvesting separates microalgal cells from the culture medium using methods such as centrifugation, flocculation, filtration, flotation, or advanced techniques like ultrasound and electrolysis. The biomass is then dried to reduce moisture for improved stability. Cell disruption is essential for metabolite extraction and can be achieved through mechanical (e.g., bead milling, homogenization), chemical (e.g., osmotic shock), or enzymatic methods. Extraction methods include solvent-based techniques. Purification is completed using chromatography, ultrafiltration, or crystallization to isolate and refine the target compounds.
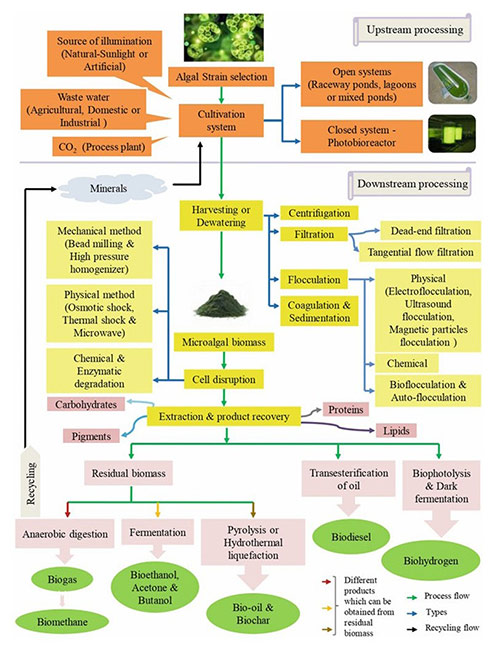
Figure 2. Different DSP units involved in microalgal biometabolite production. (Banerjee et al ., 2024)
Case 2: Serial fractionation of spent brewer’s yeast protein hydrolysate by ultrafiltration
Marson et al. , 2022. doi:10.1016/j.jfoodeng.2021.110737
This study developed a 3-step membrane filtration process to separate peptides from sugars and RNA in spent brewer’s yeast (SBY) hydrolysate. Two membrane sequences were tested (50/8/1 kDa and 15/8/1 kDa), with the 15 kDa membrane showing better performance. The process successfully enriched small peptides (<1 kDa), increasing peptide purity up to 2.7-fold for sugars and 1.7-fold for RNA. The resulting protein-rich ingredients had low RNA content (as low as 1.4%) and diverse peptide profiles, showing strong potential for use in food and pharmaceutical applications.
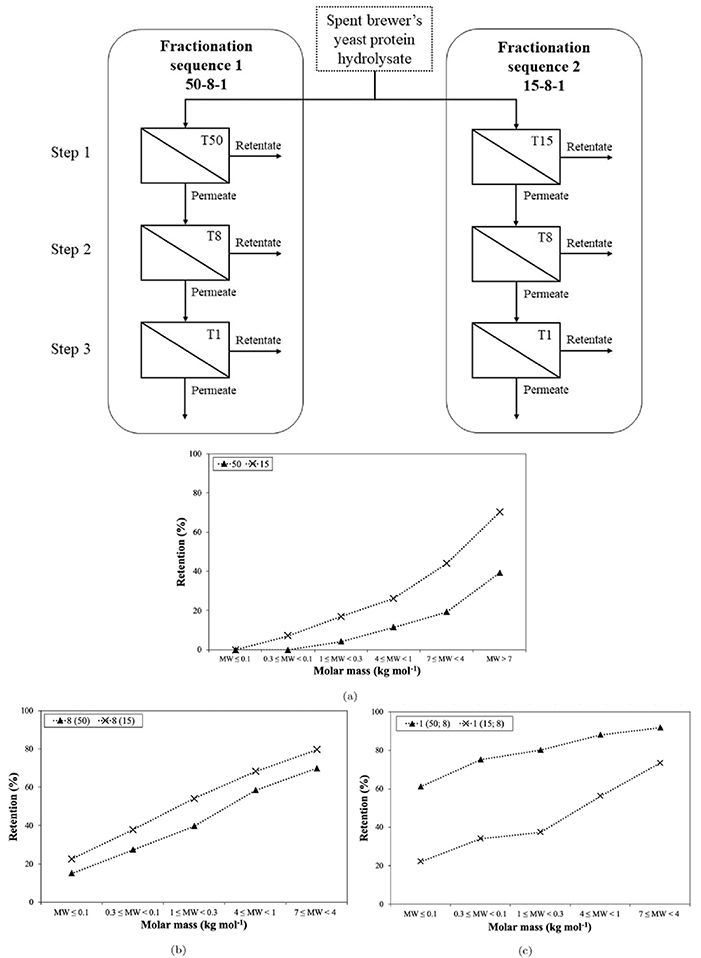
Figure 3. Top: Experimental scheme of UF sequences and steps used in the fractionation of spent brewer’s yeast protein hydrolysate using ceramic membranes of 50 (T50), 15 (T15), 8 (T8) and 1 (T1) kg mol molecular weight cut-off, respectively. Bottom: Retention versus molar mass of peptide fractions during UF fractionation in steps 1 (a), 2 (b) and 3 (b) using 50, 15, 8 and 1 kDa MWCO membranes. (Marson et al. , 2022)
Client Feedback on Our Downstream Processing Capabilities
"Creative BioMart played a critical role in helping us scale up our downstream purification process for a recombinant fusion protein. Their team quickly optimized affinity and ion exchange steps, significantly improving our yield and purity. The detailed development report they provided was regulatory-ready and extremely helpful for our IND submission."
— R&D Director | Mid-Sized Biopharma Company
"We partnered with Creative BioMart for downstream development of our viral vector-based vaccine. Their team integrated ultrafiltration and chromatography steps seamlessly, and their expertise in viral clearance validation ensured we met all GMP standards. Their responsiveness and technical insight exceeded our expectations."
— Process Development Manager | Global Vaccine Manufacturer
"We had a limited quantity of a novel enzyme candidate and needed precise downstream development to support preclinical studies. Creative BioMart customized the purification workflow and minimized product loss while maintaining functional activity. Their technical support and problem-solving approach were truly impressive."
— Principal Scientist | Academic-Industry Research Center
"As a small biotech, we needed a partner who could handle both the technical complexity and regulatory requirements of our glycoprotein product. Creative BioMart not only developed an efficient purification process but also performed thorough viral clearance studies, saving us valuable time and resources."
— Senior Scientist | Startup Focused on Rare Diseases
Frequently Asked Questions (FAQs)
-
Q: What types of molecules can you handle in downstream processing?
A: We have extensive experience with a wide range of biologics, including monoclonal antibodies, fusion proteins, enzymes, viral vectors, and glycoproteins. Each purification process is tailored to the structural and functional properties of your specific molecule. -
Q: How do you ensure high product purity and yield?
A: We apply a combination of chromatography techniques (e.g., affinity, IEX, HIC, SEC) and filtration technologies, optimizing each step based on your molecule’s characteristics. Our goal is always to balance maximum recovery with regulatory-grade purity. -
Q: Do you provide process scale-up and tech transfer support?
A: Absolutely. We develop scalable, commercially viable purification strategies and provide full documentation to ensure a smooth transition from lab-scale to pilot and production-scale environments. -
Q: What makes your downstream services different?
A: Our strengths lie in combining advanced purification platforms, a highly experienced scientific team, flexible project engagement models, and a strong focus on cost-effectiveness and regulatory compliance. We deliver customized solutions that reduce risk and accelerate your path to market. -
Q: What deliverables can I expect at the end of a project?
A: You will receive a comprehensive development report detailing all process steps evaluated, data supporting the final selected process, scale-up parameters, and justifications—ready for inclusion in regulatory submissions.
Resources
Related Services
- GMP Stable Cell Line Development Platform
- Quality Control
- Endotoxin Removal Service
- GLP-Compliant Bioprocess Services
- Integrated Drug Development & Manufacturing Bioprocess Development
Related Products
References:
- Banerjee S, Mandari V, Shalini M, Nithyashree R, Kinage C. Cost-effective downstream processing of algal biomass for industrial-scale biofuels production. In: Bharadvaja N, Kumar L, Pandit S, Banerjee S, Anand R, eds. Recent Trends and Developments in Algal Biofuels and Biorefinery . Springer Nature Switzerland; 2024:239-262. doi:10.1007/978-3-031-52319-9_11
- Jungbauer A. Continuous downstream processing of biopharmaceuticals. Trends in Biotechnology . 2013;31(8):479-492. doi:10.1016/j.tibtech.2013.05.011
- Marson GV, Lacour S, Hubinger MD, Belleville MP. Serial fractionation of spent brewer’s yeast protein hydrolysate by ultrafiltration: A peptide-rich product with low RNA content. Journal of Food Engineering . 2022;312:110737. doi:10.1016/j.jfoodeng.2021.110737
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

