High Concentration Formulation (HCF) Technology
Creative BioMart offers specialized High Concentration Formulation (HCF) services to overcome solubility and stability challenges for protein therapeutics. Our proprietary amino acid-based formulation platform enables the development of stable, low-viscosity biologic formulations at concentrations exceeding 100 mg/mL, suitable for subcutaneous or ready-to-use injection formats. Using a combination of high-throughput screening, excipient optimization, and deep protein stability analysis, we transform low-solubility or lyophilized biologics into highly concentrated liquid formulations—without compromising activity or integrity. Whether you are repurposing an IV biologic for subcutaneous use or conducting early-stage feasibility studies, our HCF technology streamlines your path to clinical success.

Background: The Challenge of High Concentration Protein Formulation
High Concentration Formulation (HCF) technology refers to the development of pharmaceutical formulations with high concentrations of active ingredients, typically proteins or monoclonal antibodies (mAbs). Formulating protein therapeutics at high concentrations—often above 100 mg/mL—is critical for enabling subcutaneous delivery and reducing patient burden. However, many biologics suffer from solubility limits, aggregation, or high viscosity when concentrated, which can lead to loss of bioactivity and hinder manufacturability.
Traditional stability testing methods frequently fall short in this context. Some lack the dynamic range needed to evaluate stability across a concentration gradient, while others may induce aggregation through physical stress alone. Consequently, a more refined, protein-specific approach is required.
Figure 1. Strategies of high-concentration protein formulations. (Garidel et al., 2017)
Creative BioMart addresses these challenges with a proprietary amino acid stabilization platform, developed through years of experience in protein production, formulation, and analytics. Our approach enables formulation teams to explore new concentration thresholds with confidence.
What We Offer–Our HCF Technology Solutions
Service Procedure
-
Duration: 1 week
1
Initial Evaluation
- Sample receipt and basic analytical characterization
- Solubility and aggregation testing at higher concentrations
- Early feasibility assessment for HCF development
-
Duration: 2–4 weeks
2
Formulation Screening
- High-throughput screening (HTS) of excipient/salt/amino acid matrices
- Parallel evaluation of physical stability (aggregation, viscosity, etc.)
- Identification of promising stabilizer candidates
-
Duration: 3–6 weeks
3
Optimization & Validation
- Fine-tuning of leading formulations
- Functional/activity assays
- Accelerated stability studies
- Real-time stability setup (ongoing if required, but not gating for final formulation selection)
-
Duration: 1 week
4
Delivery & Support
- Compilation of formulation protocols and analytical data
- Optional support for scale-up, lyophilization strategy, or documentation for regulatory submissions
Service Details
|
Item |
Details |
|---|---|
|
Protein Concentration Goals |
Formulations typically >100 mg/mL in <1.5 mL volume |
|
Excipient Screening |
Custom combinations of salts, sugars, amino acids, polymers |
|
Protein Types Handled |
Antibodies, enzymes, fusion proteins, cytokines, and more |
|
Stability Assessment Tools |
|
|
Applications |
|
Advantages of Partnering with Us for High Concentration Protein Formulation
- Proprietary Stabilizer Technology: Our amino acid-based platform reduces aggregation and viscosity while preserving protein conformation.
- Multi-Criteria Stability Evaluation: We assess more than just solubility—we protect structure, activity, and delivery viability.
- Tailored to Your Protein: Each formulation is designed based on the unique biophysical properties of your molecule.
- Rapid Feasibility Screening: Quick turnaround on early-stage HCF evaluation.
- Transformational Applications: Enable subcutaneous dosing, simplify logistics with liquid formulations, and reduce injection volumes.
- Formulation + Manufacturing Expertise: Backed by years of protein production and analytical experience.
Real-World Applications of HCF Technology in Biopharmaceutical Projects
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Managing surfactant stability in HCF technology
Roy et al., 2021. doi:10.1016/j.xphs.2021.05.012
In high concentration monoclonal antibody (mAb) formulations (>100 mg/mL), polysorbates (PS20/PS80) are widely used as stabilizing surfactants. However, trace host cell proteins (HCPs) can retain enzymatic activity and gradually degrade these surfactants, leading to fatty acid accumulation and the formation of subvisible particles during storage. A case study revealed sudden particle formation in an HCF drug product stored at 5 °C. The investigation highlighted the importance of polysorbate concentration and composition, and demonstrated that alternative surfactants, solubilizers, and HCP management strategies are effective in preventing particle formation and improving long-term HCF stability.
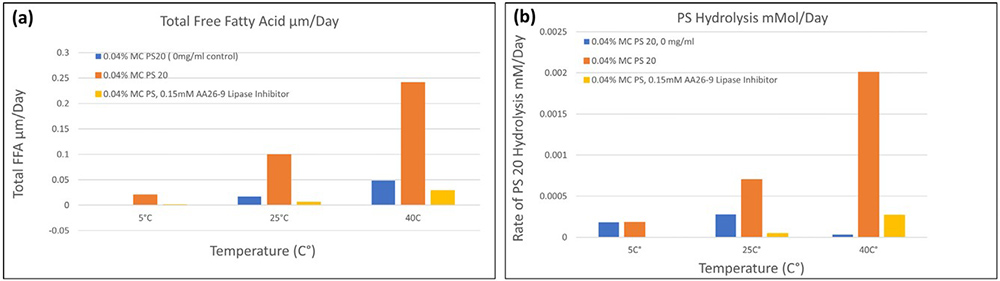
Figure 2. Effect of esterase inhibitor AA26-9 on mAb formulation stability (>100 mg/mL, L-histidine, 0.04% PS20, pH 5.6). (a) FFA accumulation and (b) PS20 hydrolysis at 5 °C, 25 °C, and 40 °C over 2 years, 9 months, 3 months respectively. (Roy et al., 2021)
Case 2: Overcoming peptide formulation challenges with HCF technology
Hu et al., 2023. doi:10.3390/ph17010015
Subcutaneous delivery of therapeutic peptides requires high concentration formulations (HCF) due to limited dosing volumes, but this presents challenges such as low solubility, high viscosity, and instability. A novel approach using low-shear resonant acoustic mixing enables the preparation of stable peptide nanosuspensions without harsh solvents or lipids. This method avoids the degradation seen with traditional high-shear processes and produces formulations with low viscosity and high stability, even at concentrations above 100 mg/mL. A cyclosporine nanosuspension demonstrated sustained release in rats, highlighting nanosuspension technology as a promising HCF solution for peptide-based therapeutics.
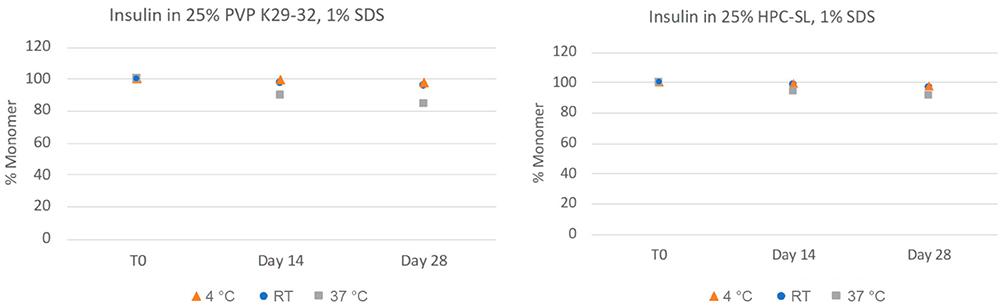
Figure 3. Chemical stability of insulin nanosuspensions in two selected formulations (wt% to insulin): (1) 25% PVP K29-32, 1% SDS, and (2) 25% HPC-SL, 1% SDS, prepared using resonant acoustic milling measured via reverse-phase chromatography. (Hu et al., 2023)
Client Success Stories Using Our High Concentration Formulation Expertise
"We partnered with Creative BioMart to convert one of our IV-administered monoclonal antibodies into a high-concentration subcutaneous formulation. Our protein had severe viscosity issues above 90 mg/mL, but their proprietary amino acid stabilizer platform solved it—resulting in a stable 120 mg/mL formulation with excellent syringeability. Their team was efficient, responsive, and transparent throughout the 10-week project."
— Director of Formulation Development | Global Biopharmaceutical Company
"Creative BioMart’s HCF service was a game-changer for our enzyme-based therapy. We needed a concentrated, ready-to-use formulation for field deployment, and they delivered a stable liquid format at 100 mg/mL with no loss in enzymatic activity. The project included aggregation screening and accelerated stability data, which were critical for our investor pitch and IND planning."
— Senior Scientist | Early-Stage Biotech Startup
"Our biosimilar version of a blockbuster antibody required matching high-concentration subcutaneous delivery. Creative BioMart helped us achieve a formulation at 150 mg/mL with minimal viscosity, enabling easy prefilled syringe development. Their high-throughput excipient screening was extremely thorough, and the documentation they provided for regulatory filing was top-notch. It saved us at least two months of internal work."
— R&D Manager | Mid-Sized Biosimilar Manufacturer
"We were exploring high-dose delivery for a cytokine therapeutic in a murine model and needed a fast turnaround formulation at 110 mg/mL. Creative BioMart’s team not only provided the formulation but also helped us assess stability using multiple analytical techniques, including DLS and circular dichroism. The protein retained 95% activity after 30 days."
— Principal Investigator | Government-Funded Research Institute
Frequently Asked Questions About High Concentration Protein Formulation Services
-
Q: What types of proteins can be formulated using your HCF technology?
A: Our platform supports a wide range of biologics, including monoclonal antibodies, enzymes, cytokines, fusion proteins, and more. We customize each formulation based on the unique biophysical and stability characteristics of your protein. -
Q: What protein concentrations can you achieve?
A: We routinely formulate proteins at concentrations above 100 mg/mL, and in many cases up to 150 mg/mL or higher, depending on the protein’s solubility and stability. Our proprietary amino acid stabilizers help reduce aggregation and viscosity at these elevated levels. -
Q: How do you reduce viscosity and aggregation in high concentration formulations?
A: We use a proprietary combination of amino acid excipients and perform high-throughput screening of salts and other stabilizers. These components are specifically chosen to minimize viscosity, inhibit aggregation, and preserve protein conformation without compromising activity. -
Q: Can you help transition our IV formulation to a subcutaneous one?
A: Absolutely. One of the primary applications of our HCF technology is converting intravenous (IV) biologics into subcutaneous (SC) formulations. This enables lower injection volumes, enhanced patient compliance, and greater commercial flexibility. -
Q: How long does the HCF development process take?
A: The full process typically takes 6 to 12 weeks, depending on the complexity of the protein and the level of formulation optimization and validation required. We also offer quick feasibility studies to evaluate HCF potential before full-scale development. -
Q: Do you offer analytical support as part of the formulation service?
A: Yes. We provide comprehensive analytical characterization, including assessments of aggregation, degradation, viscosity, bioactivity, and protein conformation using techniques like DLS, SEC, CD, and ELISA. Stability under real-time and accelerated conditions is also evaluated. -
Q: Can your formulations be used for regulatory submissions or clinical studies?
A: Yes. Our HCF service includes detailed documentation and data packages that support regulatory filings, preclinical testing, and early clinical development. We can also collaborate on scaling and tech transfer for manufacturing.
Resources
Related Services
Related Products
References:
- Garidel P, Kuhn AB, Schäfer LV, Karow-Zwick AR, Blech M. High-concentration protein formulations: How high is high? European Journal of Pharmaceutics and Biopharmaceutics . 2017;119:353-360. doi:10.1016/j.ejpb.2017.06.029
- Hu C, Zang N, Tam YT, et al . A new approach for preparing stable high-concentration peptide nanoparticle formulations. Pharmaceuticals . 2023;17(1):15. doi:10.3390/ph17010015
- Roy I, Patel A, Kumar V, et al . Polysorbate degradation and particle formation in a high concentration mab: formulation strategies to minimize effect of enzymatic polysorbate degradation. Journal of Pharmaceutical Sciences . 2021;110(9):3313-3323. doi:10.1016/j.xphs.2021.05.012
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

