Quality Control
Background on Quality Control in Biologics and Pharmaceutical Manufacturing
Quality control (QC) a is fundamental and indispensable aspect of the pharmaceutical industry, ensuring that medications meet stringent standards of safety, efficacy, and consistency. Given the critical nature of pharmaceutical products in healthcare, QC plays a vital role in safeguarding public health and maintaining regulatory compliance.
In the context of the pharmaceutical industry, quality control encompasses a comprehensive suite of processes and procedures designed to monitor and verify the quality of raw materials, intermediates, and finished products throughout the entire manufacturing lifecycle. From the initial receipt of raw materials to the final release of a pharmaceutical product, QC activities are meticulously integrated at every stage to identify and mitigate potential quality risks.
Core QC Elements
- Raw Material Testing : Ensures identity, purity, and potency to prevent contamination at the source.
- In-Process Testing : Monitors key production stages to maintain consistency and product integrity.
- Finished Product Testing : Verifies final product specifications, including potency, stability, and packaging.
- Stability Testing : Assesses shelf life and storage conditions through long-term and accelerated studies.
- Documentation & Auditing : Maintains traceable QC records and supports regulatory compliance via regular audits.
At Creative BioMart , we recognize that the faster a drug clears approval processes and passes QC testing, the sooner it can reach patients who depend on it. Our comprehensive QC Solutions help our clients navigate this journey efficiently—without compromising on quality.
Our Capabilities on Pharmaceutical Quality Control
Creative BioMart provides end-to-end pharmaceutical quality control services covering every stage of the production lifecycle—from raw material inspection to final product verification. Our state-of-the-art QC laboratories are equipped with advanced analytical technologies and are staffed by a team of experienced scientists who are dedicated to delivering regulatory-compliant, high-quality results.
Our services support both small-molecule drugs and complex biologics, and are fully aligned with cGMP, ICH, and pharmacopeial guidelines (USP, EP, JP, BP).
- Batch release testing
- Lot release testing
- API, IMP, and finished product (FP) testing
- Compliance with global pharmacopeias
Service Procedure

Service Details
-
Raw Material and Packaging Control
- Identity, purity, and quality assessment of APIs, excipients, and packaging components
- Evaluation of contact and secondary packaging materials
- Compliance with pharmacopeial specifications
-
In-Process and Final Product Testing
- Testing during phased production to ensure quality consistency
- Final release testing including assay, dissolution, impurity profiling, and microbiological safety
-
Microbiological Quality Control
- Bioburden testing
- Sterility and endotoxin testing
- Environmental monitoring in manufacturing areas
-
Analytical Method Services
- Method Development & Validation (ICH Q2-compliant)
- Method Transfer & Remediation to align with client and regulatory requirements
- Support for both early and late-stage development programs
-
Stability & Product Development
- Stability testing under ICH conditions
- Analytical support for formulation optimization and shelf-life determination
-
Regulatory & Validation Support
- Registration testing and submission support
- Validation of cleaning processes, equipment, and analytical methods
Advanced Instrumentation & Techniques
-

HPLC
-

GC-MS
-

FTIR
-

MS
-

ICP-MS
-

XRD
Why Our QC Services Ensure Consistent Product Safety and Efficacy
- Accredited & Experienced : Fully certified QC lab with years of proven experience.
- Broad Analytical Scope : From raw materials to complex biologics and combination products.
- Regulatory Expertise : Deep understanding of global regulatory expectations (FDA, EMA, MHRA).
- Customizable Solutions : Services tailored to your molecule, modality, and development stage.
- Methodological Flexibility : Robust capabilities in method transfer, development, and validation.
- Integrated Support : Seamless collaboration between QC, stability, and release testing programs.
Case Studies: Real-World Success in Pharmaceutical Quality Control
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Multivariate quality assessment of Apocyni veneti folium
Chen et al ., 2018. doi:10.3390/molecules23030573
This study established a comprehensive quality evaluation method for Apocyni veneti folium (AVF), a widely used traditional Chinese medicine with inconsistent quality due to habitat differences and adulteration. Using UFLC-QTRAP-MS/MS, researchers simultaneously quantified 43 bioactive compounds—flavonoids, organic acids, amino acids, and nucleosides—in 41 AVF and commercial samples. Principal component analysis (PCA) and gray relational analysis (GRA) were applied to distinguish AVF from its common adulterant, Poacynum hendersonii (PHF), and assess overall quality. Results showed AVF samples had superior quality to PHF. This method offers a reliable approach for ensuring AVF’s authenticity and consistency in clinical use.
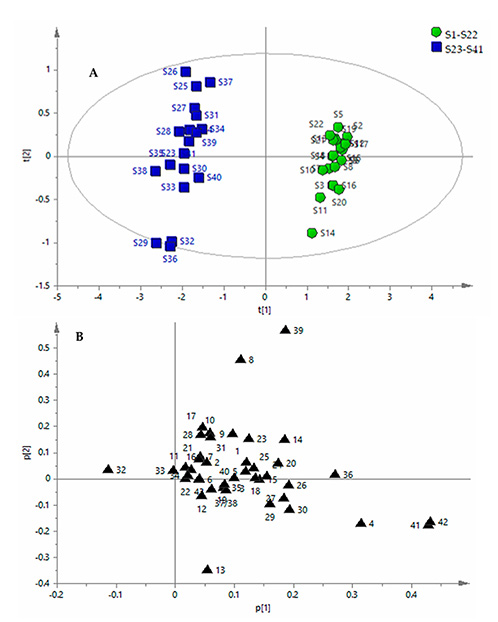
Figure 1. Multivariate statistical analysis of 41 samples of Apocyni veneti folium (AVF) and Poacynum hendersonii (PHF). Principal component analysis (PCA) scores plot (A) and PCA loading plot (B) of samples. (Chen et al. , 2018)
Case 2: Management of validation of HPLC method for determination of acetylsalicylic acid impurities in a new pharmaceutical product
Kowalska et al. , 2022. doi:10.1038/s41598-021-99269-x
This study focused on validating an HPLC method for determining salicylic acid and unknown impurities in a new tablet formulation containing 75, 100, or 150 mg of acetylsalicylic acid and 40 mg of glycine. Separation was achieved using a Waters Symmetry C18 column with a mobile phase of orthophosphoric acid, acetonitrile, and water (2:400:600, V/V/V), and detection at 237 nm. The method was validated according to ICH guidelines, assessing linearity, precision, accuracy, specificity, robustness, and stability. Results confirmed the method is linear, precise, and accurate within a 0.005–0.40% range for salicylic acid relative to acetylsalicylic acid content.
What Clients Say About Our Pharmaceutical Quality Control Services
“Creative BioMart supported our method transfer and validation for a modified-release tablet entering Phase III. Their team quickly adapted our legacy HPLC assay, tightened precision parameters, and handled full validation under ICH Q2 guidelines. Their documentation made our regulatory submission process far smoother than expected.”
— Director of Analytical Development | Mid-Sized Pharmaceutical Company
“We faced a bottleneck with our accelerated stability study for a topical formulation. Creative BioMart stepped in with fast onboarding, clear protocols, and excellent temperature/humidity-controlled storage. Their timely data helped us file our IND extension without delay.”
— Regulatory Affairs Manager | Dermatology-Focused Biotech Firm
“During scale-up, we needed intensive raw material QC, including elemental impurity testing via ICP-MS. Creative BioMart delivered precise and reproducible results, and even flagged a borderline contaminant we’d missed internally. That intervention likely saved us from a costly deviation.”
— Head of Quality | CDMO Specializing in Injectables
“Creative BioMart helped us with complete compendial testing for a combination product with a novel excipient. Their team cross-referenced multiple pharmacopeias and helped validate our packaging integrity testing. Their scientific rigor made them feel like an extension of our own QC department.”
— R&D Director | Global Generic Drug Company
FAQs on Quality Control Testing in Drug Manufacturing Processes
-
Q: Do you provide compendial testing (USP, EP, JP)?
A: Yes, we perform full compendial testing in accordance with USP, EP, JP, and BP standards. -
Q: Can you assist with method transfer from our internal labs?
A: Absolutely. We offer method transfer, bridging, and remediation services, with full documentation support. -
Q: How do you ensure data integrity and regulatory compliance?
A: Our QC labs operate under strict cGMP and ALCOA+ principles, and our data systems are audit-ready and 21 CFR Part 11 compliant. -
Q: What’s your turnaround time for routine QC testing?
A: Turnaround times vary by test, but standard raw material and batch release tests can often be completed within 5–10 business days. -
Q: Do you offer support for ICH stability studies?
A: Yes, we offer long-term, accelerated, and stress condition stability testing aligned with ICH guidelines.
Resources
Related Services
- Downstream Processing Development
- Biosimilar Comparability Studies
- cGMP Cell-Based Potency Assays
- Pharmaceutical Stability Analysis
- GLP-Compliant Bioprocess Services
- Biopharmaceutical Process and Product Related Impurities Analysis
Related Products
References:
- Chen C, Liu Z, Zou L, et al . Quality evaluation of Apocyni veneti folium from different habitats and commercial herbs based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Molecules . 2018;23(3):573. doi:10.3390/molecules23030573
- Kowalska M, Woźniak M, Kijek M, Mitrosz P, Szakiel J, Turek P. Management of validation of HPLC method for determination of acetylsalicylic acid impurities in a new pharmaceutical product. Sci Rep . 2022;12(1):1. doi:10.1038/s41598-021-99269-x
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions


