What is PARK2 Protein?
Background of PARK2 Protein
The PARK2 protein (also known as Parkin), was first discovered in 1997 while investigating families with early-onset Parkinson's disease (PD). Its corresponding gene has been localized to the long arm of chromosome 6, at 6q25.2-q27. A product of the PARK2 gene, this protein holds prime significance in the arena of neurogenerative research, primarily due to its role in the autosomal recessive juvenile form of PD.
PARK2 belongs to a family of proteins classified as E3 ubiquitin ligases, known for their ubiquitylation capacity, a crucial aspect for cellular homeostasis. Structurally, it comprises a ubiquitin-like domain at its N-terminus and two RING finger domains separated by an in-between-RING (IBR) domain, which together facilitates its role in ubiquitin-proteasome system (UPS).
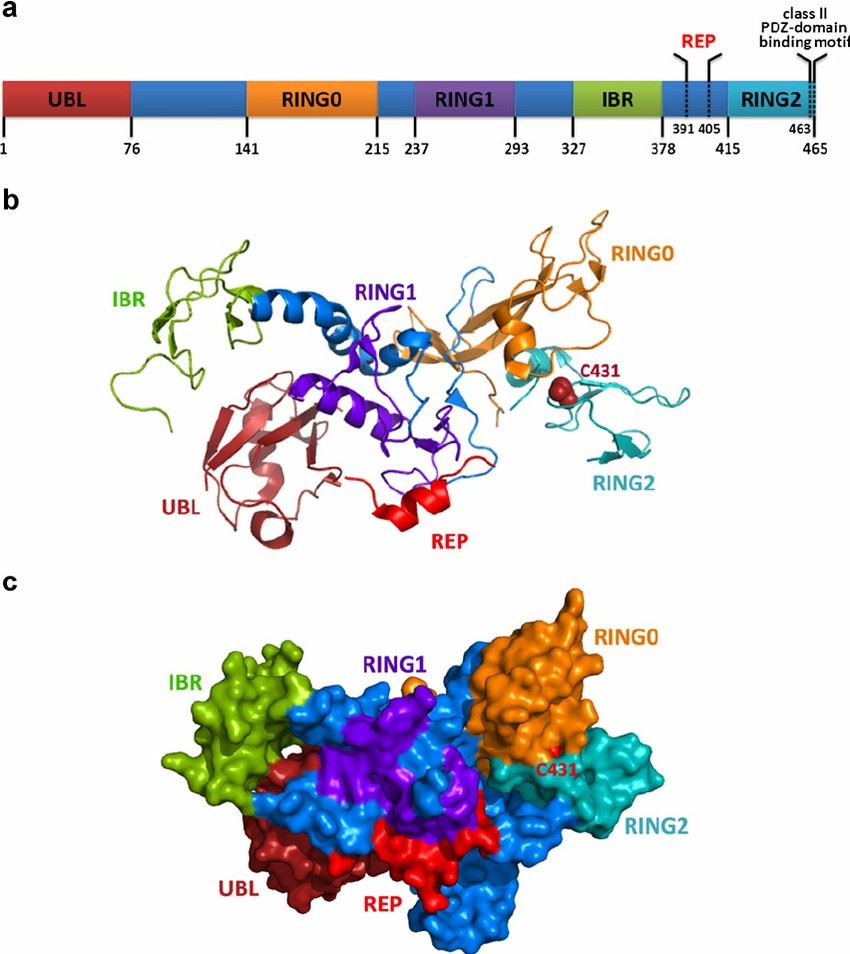
Fig 1. Schematic and spatial illustrations of PARK2 structure (Xu, Liang et al. 2013)
Function of PARK2 protein
Diving into the function of this protein, PARK2 is largely involved in the tagging of unwanted or damaged proteins with ubiquitin molecules. This 'tagging' signals the proteasomal machinery to degrade the marked proteins, thereby maintaining a healthy cellular environment. Furthermore, PARK2's role extends to maintaining mitochondrial integrity by marking damaged mitochondria for autophagy – a process known as mitophagy.
PARK2 protein related signal pathway
Recent studies have focused extensively on the signal pathway related to the PARK2 protein. Damage to mitochondria causes a decrease in their membrane potential, which triggers the translocation of the PARK2 protein to the outer mitochondrial membrane. Here, PARK2 ubiquitinates various outer membrane proteins, a significant signal for specialized machinery to induce mitophagy. Notably, PTEN-induced putative protein kinase 1 (PINK1), another protein linked to PD, plays a vital role in recruiting PARK2 to the damaged mitochondria, solidifying the interplay of these proteins in this signal pathway.
PARK2 protein related diseases
The essential contribution of PARK2 to cellular and mitochondrial homeostasis underlines its association with various diseases. Most notably, mutations in the PARK2 gene are one of the foremost genetic links to PD, where they give rise to defective parkin proteins resulting in compromised mitochondrial quality control. Additionally, PARK2 mutations and deficiencies have been implicated in various cancers, suggesting the protein's role in tumor suppression. Associations with heart disease, diabetes, and several neurodegenerative disorders further highlight PARK2's multifaceted significance in human health and disease.
PARK2 protein's applications in biomedical
Given the pivotal role of PARK2 in maintaining cellular homeostasis and its interplay with various diseases, this protein has opened up unprecedented opportunities in the realm of biomedicine. The application of PARK2 protein understanding in therapeutic development shows promise in PD treatment. Mutations in PARK2 gene leading to non-functional parkin have been identified as a potential therapeutic target. Vector-based parkin gene therapy, where a healthy parkin gene is introduced to compensate for the non-functional one, has shown potential in preclinical models of PD.
Moreover, unveiling PARK2-associated signaling pathways and its role in tumorigenesis has implications on cancer therapy. Exploring various ways of boosting PARK2 activity could have potential benefits in cancer prevention and treatment. Lastly, PARK2's role in cardiovascular health, diabetes, and other neurodegenerative conditions echoes its potential as a valuable therapeutic target in these diseases.
The elucidation of PARK2's background, function, signaling pathway, associated diseases, and biomedical applications unveils a protein of enormous significance. Whereas our understanding of this protein has made great strides over the past two decades, much remains to be learned about its intricacies. As scientific investigation into the PARK2 protein deepens and broadens, the character of this compelling protein winds its way to promising, untapped therapeutic potentials.
Our Featured Products
| Cat.No. | Product Name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| PARK2-1529H | Recombinant Human PARK2, His-tagged | Human | E.coli | His |
| PARK2-231H | Recombinant Human PARK2, GST-tagged | Human | Sf9 Insect Cell | GST |
| PARK2-11H | Recombinant Human PARK2 protein, His-tagged | Human | HEK293 | His |
| PARK2-6497M | Recombinant Mouse PARK2 Protein, His (Fc)-Avi-tagged | Mouse | HEK293 | His (Fc)-Avi |
| ARK2-3935R | Recombinant Rat PARK2 Protein, His (Fc)-Avi-tagged | Rat | HEK293 | His (Fc)-Avi |
| ARK2-772C | Recombinant Cynomolgus PARK2 Protein, His-tagged | Cynomolgus Monkey | Mammalian Cell | His |
Reference
- Xu, Liang & Lin, Dechen & Yin, Dong & Koeffler, H. (2013). An emerging role of PARK2 in cancer. Journal of molecular medicine (Berlin, Germany). 92. 10.1007/s00109-013-1107-0.

