What is PLA2G1B Protein?
The Phospholipase A2 Group 1B (PLA2G1B) protein is a crucial and complex biomolecule that plays multiple important roles in numerous physiological and pathological processes.
Background and Discovery of PLA2G1B Protein
The PLA2G1B protein was discovered as one of the several types of phospholipase A2 (PLA2) isoforms, a family of enzymes that catalyzes the hydrolysis of the sn-2 ester bonds of cellular glycerophospholipids to produce free fatty acids and lysophospholipids. The group has diverse physiological functions in a variety of organismal areas from cell membrane homeostasis to inflammation, coagulation, and apoptosis.
The gene that encodes PLA2G1B, also called PLA2G1B, is located on chromosome 12q23.3 in humans. The protein is encoded by the single-copy gene in most individuals, and PLA2G1B consists of several domains, including a catalytic domain, a proline-rich domain, a C2 domain, and a long C-terminal tail, with a total of 147 amino acids.
What Is The Structure of PLA2G1B Protein?
The structure of PLA2G1B protein is of great interest to scientists due to its role in lipid metabolism and regulation. It has a unique molecular structure with a typical phospholipase fold. It includes a seven-stranded anti-parallel beta-sheet capped by three alpha-helices on one side and a single helix on the other. This configuration offers a binding groove for accessing the substrate diacyl glycerylphosphoethanolamine and a calcium-binding site.
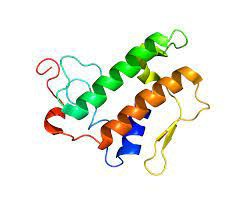
(from Wikipedia)
What Is The Function of PLA2G1B Protein?
The primary function of the PLA2G1B protein is to catalyze the hydrolysis of phospholipids in the sn-2 position, playing an essential role in lipid digestion and absorption. This hydrolysis process is crucial in generating arachidonic acid, a precursor for eicosanoids that play a significant role in inflammation, platelet aggregation, and other physiological responses. The ability to hydrolyze dietary phospholipids by PLA2G1B plays a role in nutrition absorption and metabolism.
PLA2G1B protein related signal pathway
PLA2G1B protein is involved in various signaling pathways, primarily the Phospholipase-D signaling pathway. This pathway plays an essential role in several biological processes, including cell growth, signaling, and survival. Dysregulation of this pathway can lead to a wide range of pathological conditions, such as cancer, neurodegenerative diseases, and cardiovascular diseases.
PLA2G1B protein related diseases
Research has shown that PLA2G1B protein is associated with several diseases. It has been implicated in obesity and insulin resistance due to its role in lipid metabolism. Conditions like myocardial infarction, stroke, and atherosclerosis are also connected to PLA2G1B due to its involvement in the inflammatory response and coagulation process. Moreover, it was found to be involved in colorectal cancer, although the exact mechanism of its contribution to the disease pathophysiology is yet to be fully elucidated.
PLA2G1B protein's applications in biomedical
Over the years, the PLA2G1B protein has seen a significant and growing interest in biomedical applications. The protein has emerged as a potential therapeutic target in various disease models, particularly in inflammatory and metabolic disorders. In drug discovery, PLA2G1B inhibitors have shown promise due to their ability to mitigate obesity and insulin resistance. Moreover, the key role PLA2G1B plays in lipid absorption and hydrolysis has opened possibilities in nutritional therapy, where altering its activity could potentially modify absorbing dietary fats, impacting weight management and cardiovascular health.
To sum up, while substantial strides have been made in understanding the complexity of PLA2G1B protein, continuous exploration is required. From its unique structure to complex functions, signaling pathways, and association with numerous diseases, PLA2G1B has indeed proven to be an essential player in human health and disease. As we make headway into understanding PLA2G1B more thoroughly, it's possible that this protein could provide an avenue for developing more effective therapies targeting a range of diseases.
Our Featured Products
| Cat.No. | Product Name | Species | Source (Host) | Tag | |
|---|---|---|---|---|---|
| PLA2G1B-1751H | Recombinant Human PLA2G1B, His-tagged | Human | E.coli | His | |
| PLA2G1B-171H | Recombinant Human PLA2G1B protein, His-tagged | Human | HEK293 | His | |
| PLA2G1B-1878H | Recombinant Human PLA2G1B Protein, MYC/DDK-tagged | Human | HEK293 | Myc/DDK | |
| PLA2G1B-3867H | Recombinant Human PLA2G1B Protein, His (Fc)-Avi-tagged | Human | HEK293 | His (Fc)-Avi | |
| Pla2g1b-1185M | Recombinant Mouse Pla2g1b, His tagged | Mouse | Human Cell | His | |
| Pla2g1b-4898M | Recombinant Mouse Pla2g1b Protein, Myc/DDK-tagged | Mouse | HEK293T | Myc/DDK |

