The Development journal published a research paper online titled “In situ regeneration of inner hair cells in the damaged cochlea by temporally regulated coexpression of Atoh1 and Tbx2”. The research was completed by Liu Zhiyong, a research group of the Center for Excellence and Innovation in Brain Science and Intelligent Technology (Institute of Neuroscience) of the Chinese Academy of Sciences, and the Shanghai Brain Science and Brain Research Center (Shanghai Brain Center). The research team constructed a mouse model of specific damage to cochlear inner hair cells, and based on this model, transformed juvenile cochlear support cells into new inner hair cells, thereby achieving in situ regeneration of damaged endogenous inner hair cells in the cochlea. Comprehensive and detailed transcriptomic, morphological, and electrophysiological analyses were conducted on the new inner hair cells.
Our Featured Products
Cat. No. |
Product Name |
Source |
Species |
Tag |
| ATOH1-3202H | Recombinant Human ATOH1 protein, His-tagged | E.coli | Human | His |
| ATOH1-2097M | Recombinant Mouse ATOH1 Protein | Mammalian Cell | Mouse | His |
| ATOH1-367H | Recombinant Human ATOH1 protein, GST-tagged | Wheat Germ | Human | GST |
| ATOH1-368H | Recombinant Human ATOH1 protein, His-tagged | E.coli | Human | His |
| ATOH1-369H | Recombinant Human ATOH1 protein | E.coli | Human | N/A |
| TBX19-3576H | Recombinant Human TBX19 protein, His-tagged | E.coli | Human | His |
| TBX19-6509C | Recombinant Chicken TBX19 | Mammalian Cell | Chicken | His |
| TBX19-16527M | Recombinant Mouse TBX19 Protein | Mammalian Cell | Mouse | His |
The cochlea is the first level sound receptor of the auditory system, with three rows of outer hair cells, one row of inner hair cells, and different types of supporting cells arranged in an orderly manner within its Corti organs. The inner hair cells are responsible for converting sound waves into electrical signals and transmitting them to the spiral ganglion (auditory nerve). Unlike non-mammalian animals such as fish and birds, mammals lose the ability to regenerate hair cells, so damage to hair cells can lead to irreversible permanent deafness. IBCs/IPhs are two subtypes of cochlear supporting cells, which are spatially adjacent to inner hair cells and have similar cellular origins during development. Therefore, IBCs/IPhs are potential cell sources and gene manipulation targets for inner hair cell regeneration. In the early stage, our laboratory successfully transdifferentiated IBCs/IPhs into vGlut3 newborn inner hair cells in normal (undamaged) cochlea by conditionally overexpressing Atoh1 and Tbx2 in the cochlea of newborn mice. But there are still several key issues that need to be solved urgently: 1) Can new inner hair cells be regenerated in the cochlea damaged by inner hair cells? 2) How similar are the transcriptome level and electrophysiological characteristics between new inner hair cells and wild-type inner hair cells? 3) Can new inner hair cells achieve hearing recovery or partial recovery?
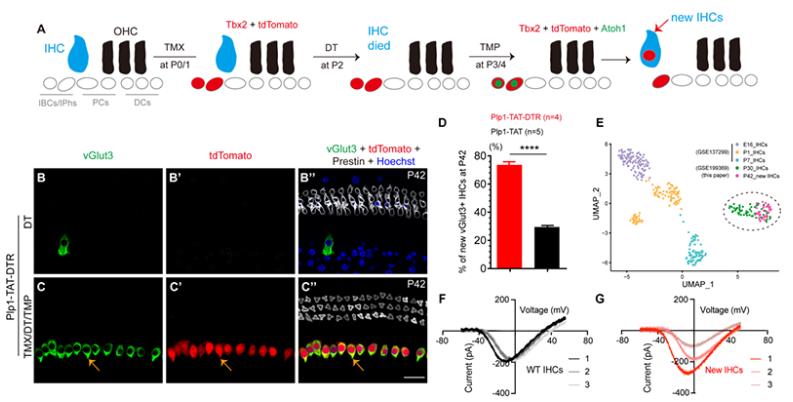
To address the aforementioned scientific issues, researchers first constructed a mouse model Fgf8-DTR/mouse with specific damage to inner hair cells. When Fgf8-DTR/is injected with diphtheria toxin (DT), it can cause 97.8% of inner hair cell death. To investigate whether damaging wild-type inner hair cells can increase the efficiency of supporting cell differentiation into inner hair cells, researchers constructed a mouse model, Plp1-CreER, that can finely regulate gene expression; Rosa26-Lexp-stop-Loxp-Tbx2-P2A-DHFR*Atoh1*DHFR-T2A-tdTomato/; Fgf8-DTR/ (abbreviated as Plp1-TAT-DTR) was divided into two groups: 1) The first group was used as the control group, and DT was only injected in P2; 2) The second group was injected with tamoxifen (TMX) in P0 and P1, DT in P2, and trimethoprim (TMP) in P3 and P4, which was used to bind DHFR and instantaneously stabilize Atoh1 expression. They were defined as the TMX/DT/TMP experimental group. The experimental results showed that the experimental group had 477.0 ± 48.0 inner hair cells, much higher than the control group (17.3 ± 4.0). Moreover, the regeneration efficiency of newly generated inner hair cells under injury (73.5% ± 2.3%) was significantly higher than that without injury (29.5% ± 1.2%). More importantly, single-cell transcriptome analysis and patch clamp electrophysiological analysis showed that new inner hair cells and wild-type inner hair cells were regenerated under scanning electron microscopy. This study demonstrates for the first time that when endogenous inner hair cells are damaged, ectopic expression of Atoh1 and Tbx2 in newborn IBCs/IPhs can regenerate new inner hair cells, which have a transcriptome and electrophysiological similarity of over 60% with endogenous inner hair cells, and the regeneration efficiency is significantly improved compared to without endogenous inner hair cell damage.
To investigate whether the regenerated inner hair cells have biological functions, researchers detected and compared the hearing of Plp1-Ai9 (TMX/TMP, positive control group), Plp1-TAT-DTR (DT, negative control group), and Plp1-TAT-DTR (TMX/DT/TMP, experimental group) through auditory brainstem response. The results showed that the hearing of the experimental group mice was not restored. To further investigate the main reasons for the inability to recover hearing, researchers used electrophysiological techniques and found that new inner hair cells do not possess mechanically sensitive electrical transduction channel currents. This discovery explains why new inner hair cells are unable to perceive external sound signals and restore hearing, providing direction for how to regenerate functional hair cells in the future.
Related Services
Protein Expression and Purification Services
Reference
Li X, Ren M, Gu Y, Zhu T, Zhang Y, Li J, Li C, Wang G, Song L, Bi Z, Liu Z. In situ regeneration of inner hair cells in the damaged cochlea by temporally regulated co-expression of Atoh1 and Tbx2. Development. 2023 Dec 15;150(24):dev201888. doi: 10.1242/dev.201888. Epub 2023 Dec 11. PMID: 38078650.