Drug Discovery Screening
Background on High-Throughput Screening in Drug Discovery
Drug discovery screening is a crucial and foundational stage in the development of new pharmaceuticals, representing the initial phase where potential therapeutic candidates are identified and evaluated. This process is essential for advancing medical research and addressing unmet clinical needs by uncovering compounds that may have therapeutic potential.
The primary goal of drug discovery screening is to identify "hits" or compounds that exhibit biological activity against a specific target, such as a protein or enzyme involved in a disease pathway. These hits are then further optimized through medicinal chemistry and biological testing to become "lead" compounds, which can eventually be developed into drug candidates. The process aims to identify compounds with high efficacy, selectivity, and safety profiles while minimizing toxicity and off-target effects.
Key Components of Drug Discovery Screening
- Target Identification & Validation: Find and confirm a biological target critical to disease.
- Compound Libraries: Use diverse collections of natural and synthetic compounds for screening.
- Screening Assays: Perform biochemical or cell-based tests, often using high-throughput automated platforms.
- Data Analysis & Hit Identification: Analyze results to find active compounds, then confirm with secondary assays.
- Follow-Up Studies: Optimize hits through chemistry and test leads for efficacy and safety in biological models.
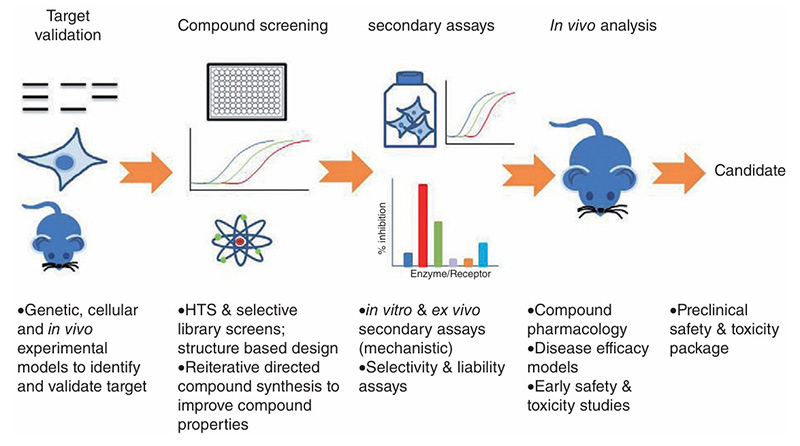
Figure 1. Drug discovery screening assays overview. (Hughes et al., 2011)
At Creative BioMart, we share our clients' passion for innovation. Our dedicated scientists work hand-in-hand with partners to accelerate drug discovery—from early high-throughput screening to late-stage preclinical validation. With robust technologies, flexible service packages, and a collaborative mindset, we aim to drive therapeutic breakthroughs from idea to reality.
Our Comprehensive Service Portfolio for Drug Discovery Screening
Creative BioMart’s Drug Discovery Screening Services are tailored to support your program across the discovery pipeline. Whether you’re screening large compound libraries, refining hits during lead optimization, or developing phenotypic assays to uncover novel mechanisms, our experienced team provides end-to-end support. Our services are fast, reproducible, scalable, and designed to fit your unique research goals.
We also specialize in Phenotypic Drug Discovery (PDD)—an approach that allows early insight into compound efficacy and toxicity by using cell-based models. PDD enables more physiologically relevant data and higher translational potential, helping you avoid costly downstream failures.
Service Procedure

Service Details
Our comprehensive screening capabilities include:
|
Type |
Details |
|---|---|
|
High-Throughput Screening (HTS) |
Rapid screening of large chemical libraries against extracellular or intracellular targets using automated platforms. |
|
Cell-Based Screening |
Functional cellular assays to evaluate compound activity in biologically relevant models—ideal for phenotypic discovery. |
|
Intracellular Target Screening |
Specialized assays targeting intracellular pathways and protein interactions to uncover potent and selective molecules. |
|
Extracellular Target Screening |
Binding and activity-based screens focused on membrane proteins, receptors, and secreted enzymes. |
|
Phenotypic Drug Discovery |
Customizable, information-rich cellular assays to identify compounds based on phenotypic outcomes rather than known targets. |
|
Custom Scaffold Discovery |
Assistance in discovering and optimizing novel chemical scaffolds that suit your biological targets and therapeutic goals. |
Why Choose Our Screening Platform for Early Drug Discovery
- Integrated Discovery Expertise: From initial screen design to lead selection, we provide strategic insight across every stage of discovery.
- Advanced Platforms: Our screening utilizes validated technologies and automation for high speed and reproducibility.
- Flexible & Customizable: Every project is tailored—no cookie-cutter solutions here.
- Proven Phenotypic Assay Development: Years of experience in designing cell-based assays that generate clinically meaningful data.
- Collaborative Partnership: We're not just a service provider—we’re an extension of your scientific team.
- End-to-End Data Support: Clear, actionable data and expert analysis to speed up your decisions.
Case Studies on Effective Drug Discovery Screening Strategies
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: C. elegans as an in vivo model system for the phenotypic drug discovery for treating paraquat poisoning
Ji et al., 2022. doi:10.7717/peerj.12866
Paraquat (PQ) is a widely used herbicide responsible for many accidental and intentional poisoning deaths, yet its exact cytotoxic mechanism and effective treatments remain unclear. To explore potential therapies, a phenotypic drug discovery (PDD) approach was applied using a newly established C. elegans model of PQ poisoning. In this model, exposure to PQ increased reactive oxygen species (ROS), malondialdehyde (MDA), and mitochondrial damage, leading to reduced lifespan and impaired functions, all of which were significantly alleviated by treatment with coenzyme Q10 (CoQ10). The protective effects of CoQ10 were further validated in a classic mouse model, where it improved survival, reduced lung ROS and MDA levels, and prevented edema and tissue damage. This study confirms the utility of the C. elegans model as a valid and scalable platform for phenotypic screening of antidotes for PQ poisoning.
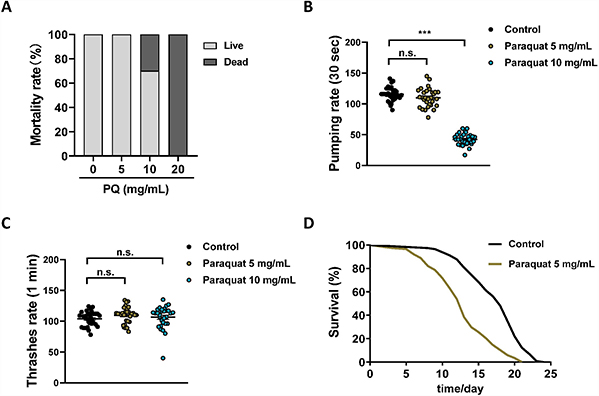
Figure 2: Establishing the C. elegans as a model for PQ poisoning. Synchronized N2 nematode populations were collected from the NGM plates at the day 1 of adulthood, subsequently exposed to different concentrations of PQ (0, 5, 10, 20 mg/mL) for 3 h. Mortality (A, n = 120), pharyngeal pumping (B, n = 30) and body bending (C, n = 30) were measured. (D) Survival curve of N2 worms after PQ (5 mg/mL) poisoning. (Ji et al., 2022)
Case 2: Systematical screening of intracellular protein targets of polyphemusin-I using E. coli proteome microarrays
Shah et al., 2021. doi:10.3390/ijms22179158
Antimicrobial peptides (AMPs) are promising alternatives to conventional antimicrobials due to their diverse mechanisms of action. Polyphemusin-I, a potent marine AMP from the American horseshoe crab, has known intracellular effects, but its specific targets were previously unknown. In this study, E. coli proteome microarrays identified 97 protein targets of polyphemusin-I, with 56 located intracellularly. Bioinformatics analyses revealed significant enrichment in nucleic acid-related functions and processes, such as RNA binding and nucleotide metabolism, suggesting nucleic acid-associated targeting. Comparative analysis with four other AMPs—two marine and three terrestrial—found limited overlap, with only nine shared targets across all five, highlighting distinct target profiles. These results reveal that polyphemusin-I uniquely targets nucleic acid-associated proteins and demonstrate differing mechanisms between marine and terrestrial AMPs, advancing our understanding of their intracellular action.
Figure 3. Images of Escherichia coli proteome microarrays and the representative protein targets of polyphemusin-I. The scan images of quadruplicate E. coli proteome microarrays probed with polyphemusin-I (left). The enlarged images of representative protein targets (right). Each red spot (in duplicate) inside the square box represents the individual protein target of polyphemusin-I identified individually from four E. coli proteome microarrays. (Shah et al., 2021)
What Researchers Say About Our Screening Capabilities
“We partnered with Creative BioMart for intracellular target screening in our oncology program. Their expertise in assay development and rapid hit validation helped us identify potent leads much faster than expected. The team’s proactive communication and scientific insight were outstanding.”
— R&D Director | Mid-Size Pharmaceutical Company
“Creative BioMart’s phenotypic screening platform was a game-changer for our neurodegenerative disease project. Their customized cell-based assays provided us with physiologically relevant data that accelerated our hit-to-lead process significantly.”
— Senior Scientist | Biotechnology Firm Specializing in CNS Disorders
“We needed high-throughput screening of a large synthetic library against a membrane receptor target. Creative BioMart not only delivered high-quality data on time but also supported us with expert data interpretation and secondary validation assays.”
— Head of Drug Discovery | Global Pharma Company
“Creative BioMart’s drug discovery screening services exceeded our expectations. Their high-throughput assay design was both innovative and scientifically rigorous, enabling us to rapidly identify novel small-molecule modulators for our oncology target.”
— Principal Investigator | Academic Research Institute in Cancer Pharmacology
FAQs on Drug Discovery Screening
-
Q: What types of targets do you support for drug screening?
A: We support a wide range of target classes including kinases, GPCRs, ion channels, enzymes, and complex intracellular protein-protein interactions. -
Q: Can you help us with custom assay development?
A: Absolutely. We specialize in customizing both target-based and phenotypic assays to suit your biological system and screening goals. -
Q: Do you offer follow-up services after screening?
A: Yes, we can support hit validation, secondary assays, mechanism-of-action studies, and even preclinical development services. -
Q: How fast is your turnaround for HTS campaigns?
A: Timelines depend on assay complexity and library size, but typical HTS projects are completed within 6–8 weeks from kickoff.
Resources
Related Services
- Cancer Biomarkers
- Protein Pathway Profiling
- Cytokine and Receptor Analysis
- Protein Engineering (Mutant Libraries)
- High-Throughput Screening
- Transporter Screening Assays
- GPCR Screening Assays
- Ion Channel Screening Assays
- Enzyme Target and Screening
- Ligand-Protein Interactions Screening Based on Gold Nanoparticles
- Protein Kinase Assay
- cGMP Cell-Based Potency Assays
Related Products
References:
- Elhadary Aya. Drug discovery, explain how lead compound delivers to the target site and claim if there are recommended antiviral drugs to COVID-19. World J Adv Res Rev. 2020;7(1):079-091. doi:10.30574/wjarr.2020.7.1.0241
- Hughes J, Rees S, Kalindjian S, Philpott K. Principles of early drug discovery. British J Pharmacology. 2011;162(6):1239-1249. doi:10.1111/j.1476-5381.2010.01127.x
- Ji P, Li H, Jin Y, Peng Y, Zhao L, Wang X. C. elegans as an in vivo model system for the phenotypic drug discovery for treating paraquat poisoning. PeerJ. 2022;10:e12866. doi:10.7717/peerj.12866
- Shah P, Chen CS. Systematical screening of intracellular protein targets of polyphemusin-I using Escherichia coli proteome microarrays. IJMS. 2021;22(17):9158. doi:10.3390/ijms22179158
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

