Breast cancer is the most common type of cancer among women. There are more than 2 million new cases of breast cancer every year. The survival rate of patients is very high, about 90%, when the tumor is still limited to breast tissue; however, when cancer cells spread beyond breast tissue and begin to form metastases in other organs, it significantly worsens the patient’s prognosis and poses great challenges to their treatment. In previous studies, researchers believed that MAF protein was directly related to the increased risk of breast cancer metastasis, but the reason behind the association between the two was not clear to researchers.
Recently, in a research report published in the journal Nature Cell Biology entitled “MAF amplification licenses ER through epigenetic remodeling to drive breast cancer metastasis”, scientists from the Barcelona Institute of Science and Technology and other institutions revealed the molecular mechanism behind MAF protein increasing the risk of metastasis in breast cancer patients through research. This research discovery may be a key step to understanding the molecular basis of breast cancer metastasis and has relevant clinical significance for the development of therapy.
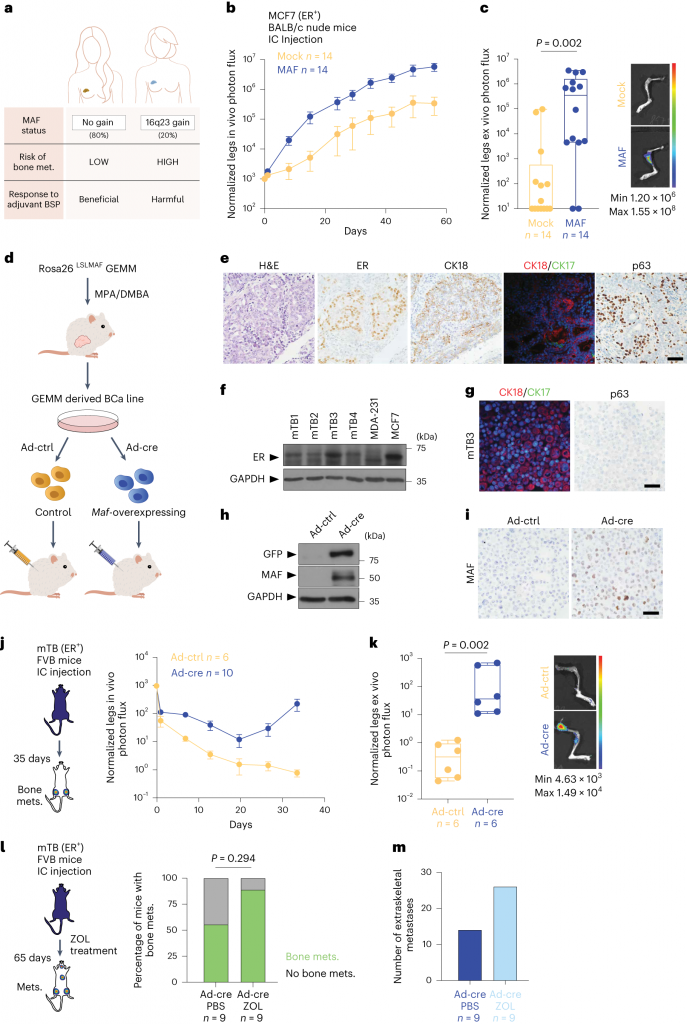
In this study, researchers revealed in detail how the MAF protein interacts with estrogen receptors to change its structure. The estrogen receptor is a key factor in the occurrence of breast cancer. This interaction may lead to DNA recombination, thus allowing genes that promote cancer metastasis to be activated, especially to respond to estrogen. These research results show that patients with high levels of MAF protein may have a greater risk of breast cancer metastasis. In this article, researchers revealed the possibility of preventing breast cancer metastasis by inhibiting the KDM1A (responsible for DNA recombination) molecule to block the activation of forward metastasis, which may provide a new perspective for the development of new breast cancer therapies. This research was carried out in cultured cells and disease animal models, and was also verified in patient samples. Previously, researchers confirmed the association between increasing the level of MAF protein and the tolerance of bisphosphonate therapy, which is used to prevent breast cancer from metastasizing to bone.
Therefore, the detection of high levels of MAF may help predict the risk of cancer metastasis, and help distinguish between breast cancer patients who benefit from bisphosphonate therapy and breast cancer patients who are not suitable for this therapy. This information is particularly important for young patients. In some cases, the treatment that can target the bone metastasis site may promote cancer metastasis to other organs, this may have a certain negative impact on mitigating overall survival. Researcher Dr. Gomis said that this research discovery may be a key step to understanding the spread of human breast cancer, and also provide new treatment opportunities for 20% of patients who cannot benefit from bisphosphonate therapy.
The detection of MAF may help identify breast cancer patients with high risk of cancer metastasis, and also help oncologists to make the most appropriate treatment choices for each case. At present, researchers have identified an element inhibitor – KDM1A protein, which is also the key to the metastasis process of breast cancer. They are testing it in clinical trials to verify its efficacy and safety. This inhibitor and related trials are independent of this research report. However, if this potential drug is proven to be effective, researchers may be able to provide certain therapeutic benefits for patients with higher levels of MAF by helping prevent the development of metastasis.
In conclusion, this study reveals the molecular basis of MAF/estrogen-mediated breast cancer metastasis, which may require genetic, epigenetic, and hormone signals from the body environment, and these signals will affect the ability of breast cancer cells to metastasize.
Related Services
Protein Expression and Purification Services
Cytokine and Receptor Analysis
Integrated Drug Development & Manufacturing Bioprocess Development
Reference
Llorente, A., Blasco, M.T., Espuny, I. et al. MAF amplification licenses ERα through epigenetic remodelling to drive breast cancer metastasis. Nat Cell Biol (2023). doi:10.1038/s41556-023-01281-y