Immune checkpoint inhibitors can stimulate the body’s anti-tumor immune system, but they can also have toxic effects called immune-related adverse events (irAEs). Colitis is a common and serious immune-related adverse event that can cause treatment interruption. As researchers did not observe a strong colitis response in laboratory mice treated with checkpoint inhibitors, Therefore, the understanding of the underlying mechanisms of intestinal immune-related adverse events is often hindered. Recently, an article titled “Microbiota-dependent activation of CD4 + T cells induces CTLA-4 blockade–associated colitis via Fcγ receptors” was published in the journal Science. In the research report, scientists from institutions such as the University of Michigan have identified a special mechanism through research that may lead to serious gastrointestinal problems caused by immunology-based cancer therapies. At the same time, researchers have also discovered a new method that can exert the anticancer effect of immunotherapy without producing uncomfortable side effects.
Medical Doctor Gabriel Nunez said that this is a great example of how understanding a particular mechanism can help us develop a more beneficial alternative therapy. Once we identify the mechanism that triggers colitis, we can develop methods to overcome this problem, while maintaining the body’s anti-tumor effect while also preventing colitis. Nowadays, immunotherapy is becoming a potential therapy for treating various types of cancer, but immune checkpoint inhibitors can also cause serious side effects, including inflammation of the digestive tract – colitis. Colitis can cause serious intestinal discomfort, and some patients may even stop cancer treatment as a result.
Our Featured Products
Cat. No. |
Product Name |
Species |
Source |
Tag |
| CTLA4-01H | Active Recombinant Human CTLA4 Protein, His-Tagged | Human | Insect Cell | His |
| CTLA4-2232H | Recombinant Human CTLA4 protein, His-tagged | Human | HEK293 | His |
| CTLA4-2233H | Recombinant Human CTLA4, Fc-His tagged | Human | Human Cell | Fc/His |
| CTLA4-255H | Recombinant Human CTLA4, His-tagged, Biotinylated | Human | HEK293 | His |
| CTLA4-257H | Active Recombinant Human CTLA4 Protein, His-Avi-tagged, Biotinylated | Human | HEK293 | His/Avi |
| CTLA4-2233HAF555 | Recombinant Human CTLA4 Protein, Fc/His-tagged, Alexa Fluor 555 conjugated | Human | HEK293 | Fc/His |
| PDCD1-172H | Active Recombinant Human PDCD1, no tag | Human | HEK293 | N/A |
| PDCD1-031HAF488 | Active Recombinant Human PDCD1 Protein, MIgG2a mFc-tagged, Alexa Fluor 488 conjugated | Human | CHO | mFc |
| PDCD1-032HAF488 | Active Recombinant Human PDCD1 Protein, hFc-tagged, Alexa Fluor 488 conjugated | Human | CHO | hFc |
The current problem faced by researchers is that when patients develop colitis, laboratory mice do not show it, so they cannot study what causes this side effect. To address this issue, researchers have developed a laboratory mouse model by injecting the microbial community of wild-captured mice into traditional mouse models. In this mouse model, when antibodies used for tumor immunotherapy were administered, mice did indeed develop colitis. Now researchers can trace this mechanism to observe what caused this reaction. The occurrence of colitis is due to the composition of the gut microbiota, which leads to excessive activation of immune T cells. At the same time, regulatory T cells that inhibit T cell activation are also eliminated in the gut, which may occur within a specific domain of immune checkpoint antibodies.
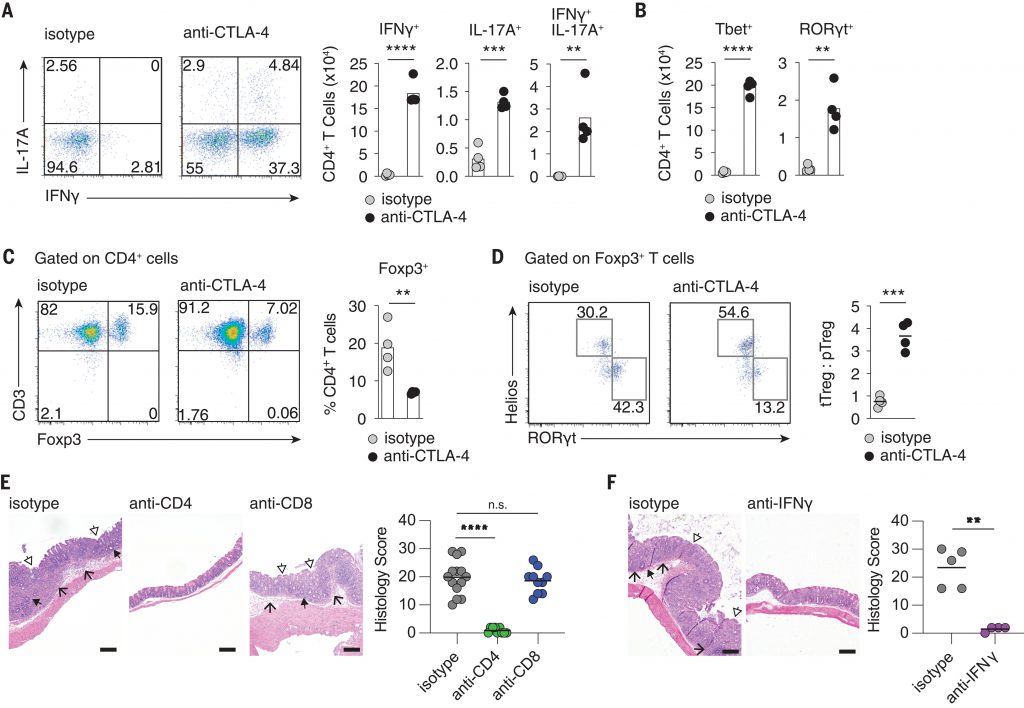
Subsequently, researchers removed this domain and found that it still elicited a strong anti-tumor response but did not induce colitis in the body. Nunez stated that previous research data has shown that the presence of certain bacteria is often associated with the body’s response to therapy, but there is no evidence to suggest that microbial communities are crucial for the occurrence of colitis.
In this study, they demonstrate for the first time that microbial communities are crucial for immune checkpoint inhibition in the body leading to colitis. To follow up on the results observed in the mouse body, researchers reanalyzed previously reported research data from cells in patients treated with immune checkpoint antibodies, which may enhance the importance of revealing regulatory T cells in inducing colitis in the body.
The next step for researchers is to conduct more research to gain a deeper understanding of the mechanisms that induce colitis in the body and to seek clinical partners to apply these research results to clinical trials.
In summary, the research findings of this article reveal a novel strategy that can slow down intestinal immune-related adverse events while retaining the anti-tumor stimulating effect produced by CTLA-4 (cytotoxic T-lymphocyte protein 4) blockade.
Reference
BERNARD C. LO, ILONA KRYCZEK,JIALI YU, et al. Microbiota-dependent activation of CD4 + T cells induces CTLA-4 blockade–associated colitis via Fcγ receptors, Science (2024). DOI:10.1126/science.adh8342