Immune Checkpoint Proteins
🧪 CD40-493H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-193 a.a.
🧪 CD200-629H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-232 a.a.
🧪 CD27-638H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-192 a.a.
🧪 CD28-640M
Source: Human Cells
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: 1-149 a.a.
🧪 CD40-174H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-193 a.a.
🧪 CD48-654H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Gln27-Ser220

🧪 Ido1-2126M
Source: E.coli
Species: Mouse
Tag: His
Conjugation:
Protein Length: 1-407 a.a.
$199.00
$398
/ 10μg
🧪 CD40-2221H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Arg193
🧪 TIGIT-2347H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Phe138
$249.00
$498
/ 50µg
🧪 Cd200-2272M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: Met1-Gly232
🧪 Cd19-3308M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: Met1-Gly287
🧪 Cd27-3273M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: Met1-Arg182
🧪 ICOS-3243H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met 1-Phe 141
🧪 CD28-3909H
Source: HEK293
Species: Human / Cynomolgus / Rhesus macaque
Tag: Fc
Conjugation:
Protein Length: 1-152 a.a.
🧪 CD28-3910H
Source: Human Cells
Species: Human
Tag: His
Conjugation:
Protein Length: 1-152 a.a.
Background
At Creative BioMart, we are proud to advance the frontiers of medicine by harnessing the power of immune checkpoint proteins. These proteins play a pivotal role in controlling immune responses, making them essential to effective immunotherapies. Our expert researchers are constantly exploring and exploiting the profound possibilities these proteins offer. Partner with us and be part of a new wave in healthcare solutions that could lead to groundbreaking therapies, powered by our cutting-edge research into immune checkpoint proteins.
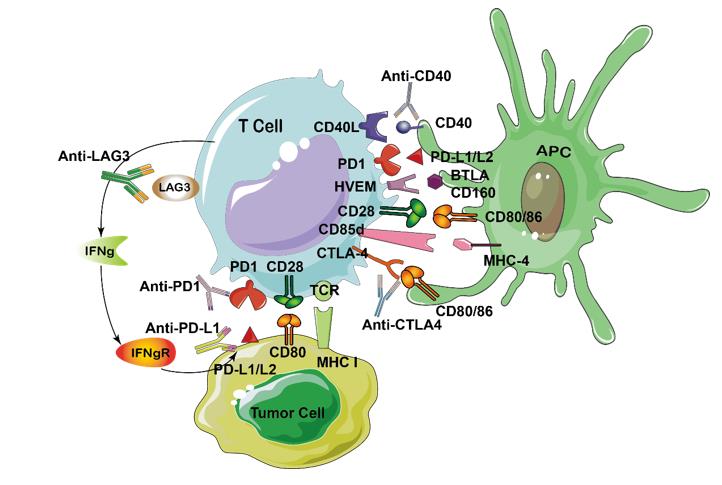
What is Immune checkpoint proteins?
Immune checkpoints are regulators of the immune system. They play a key role in maintaining immune homeostasis and preventing autoimmunity. Immune checkpoint proteins (ICP) are currently one of the most novel and promising areas of immune-oncology research. Currently, more than 3000 clinical trials of immunotherapeutic agents are ongoing with majority being ICPs.
What is Immuno-Oncology?
Immuno-oncology is an innovative area that utilizes the body's own immune system to fight cancer by modifying it to recognize the tumor as a foreign substance to the body that needs to be attacked.
There are several immuno-oncology treatments that are currently available, such as immune cell therapy (CAR-T), cytokines, monoclonal antibodies, cancer vaccines, and checkpoint inhibitors. Below is a diagram showing the immune checkpoint proteins and their inhibitor drugs.
Available Immune Checkpoint Proteins
| Immune Checkpoint Protein | Synonym | Cellular Expression | Ligand | Function |
|---|---|---|---|---|
| PD-1 (immune-suppressive checkpoint) | CD279 | Activated T cells in peripheral tissue, B cells, professional APCs, NK cells | PD-L1 (CD274, B7-H1) and PD-L2(CD273,B7-DC) | PD-1 guards against autoimmunity through a dual mechanism of promoting apoptosis (programmed cell death) in antigen-specific T-cells in lymph nodes while simultaneously reducing apoptosis in regulatory T cells (anti-inflammatory, suppressive T cells). |
| LAG-3 (immune-suppressive checkpoint) | CD223 | Activated T cells, NK cells | MHC class II (e.g. HLA-DPB1) |
Involves the unique intracellular KIEELE domain |
| TIM-3 (immune-suppressive checkpoint) | HAVcr2 | Activated T cells and tissues such as liver, small intestine, thymus, spleen, lung muscle, brain tissue | Mainly galectin-9, as well as phosphatidylserine and HMGB1 | TIM-3 is an immune checkpoint and together with other inhibitory receptors including PD-1 and LAG-3 mediate the CD8+ T-cell exhaustion. HAVCR2 has also been shown as a CD4+ Th1-specific cell surface protein that regulates macrophage activation. |
| CTLA-4 (immune- suppressive checkpoint) | CD152 | Mostly naive CD4+ and CD8+ T cells, Tregs | CD80 (B7-1) or CD86 (B7-2) | CTLA-4 is expressed by activated T cells and transmits an inhibitory signal to T cells. CTLA-4 is homologous to the T-cell co-stimulatory protein, CD28, and both molecules bind to CD80 and CD86, on antigen-presenting cells. CTLA-4 binds CD80 and CD86 with greater affinity and avidity than CD28 thus enabling it to outcompete CD28 for its ligands. CTLA4 transmits an inhibitory signal to T cells, whereas CD28 transmits a stimulatory signal. CTLA4 is also found in regulatory T cells and contributes to its inhibitory function. T cell activation through the T cell receptor and CD28 leads to increased expression of CTLA-4. |
| KIRs (immune-suppressive checkpoint) | CD158 | Mainly NK cells but also APCs and tumor-associated CTLS | MHC class I molecules (HLAs) | Inhibitory KIRs induce NK cell tolerance through licensing, in which KIR recognizes self MHC class I molecules thus preventing NK cell activation against self-tissue and auto-antigens |
| GITR (immune-activating checkpoint) | CD357 | Tregs, activated CD4+ and CD8+ T cells | GITRL | GITR is a surface receptor molecule that has been shown to be involved in inhibiting the suppressive activity of T-regulatory cells and extending the survival of T-effector cells. |
| 4-1BB(immune-activating checkpoint) | CD137 | Activated CD4+ and CD8+ T cells, Tregs, activated NK cells, DCs, neutrophils | 4-1BB-L | This receptor contributes to the clonal expansion, survival, and development of T cells. It can also induce proliferation in peripheral monocytes, enhance T cell apoptosis induced by TCR/CD3 triggered activation, and regulate CD28 co-stimulation to promote Th1 cell responses. TRAF adaptor proteins have been shown to bind to this receptor and transduce the signals leading to activation of NF-kappaB. |
Featured ICP Instruction
CTLA-4
Cytotoxic T Lymphocyte antigen 4 (CTLA-4) (CD152) and CD28 are homologous receptors expressed by both CD4+ and CD8+ T cells, which mediate opposing functions in T cell activation. Both receptors share a pair of ligands expressed on the surface of antigen-presenting cells (APCs).
The process of T cell activation is tightly regulated by costimulatory signals for full activation, and it is also regulated by co-inhibitory signals. The main costimulatory signals for T cell activation are from the B7-1 or B7-2 molecules on APC, which can bind to CD28 on T cells. After binding to their specific antigen ligand, the resulting TCR signals in conjunction with the costimulatory signals from CD28/B7 interaction lead to full activation of T cells and production of cytokines. In contrast to CD28, CTLA-4 delivers an inhibitory signal and it has a much higher affinity for B7 than CD28. Thus, CTLA-4 competes to bind to B7 and thereby prevents CD28-mediated T cell co-stimulation while inhibiting T cell activation.
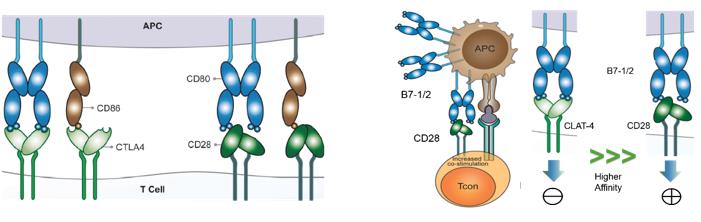
A number of different models have been proposed to explain the mechanism in the function of CTLA-4. The simplest of these involves competition between CD28 and CTLA-4 for ligand binding, which has been proposed alongside other cell-intrinsic inhibitory signaling models. CTLA-4 has a higher binding affinity for B-7 ligands than CD28, which binds these B7 proteins with an approximately 20 times greater affinity and can, therefore, outcompete CD28 for binding, leading to attenuation and termination of T cell responses and establishment of tolerance, to minimize the development of autoimmunity.
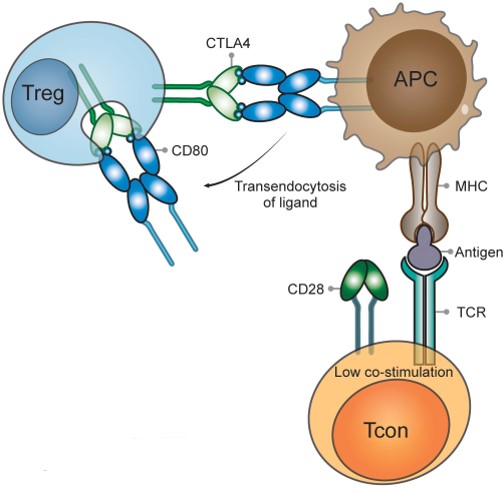
While CD28 is constitutively expressed on the plasma membrane and co-stimulates T cells, CTLA-4 is predominantly found in intracellular vesicles in FoxP3+ Treg cells or activated conventional T cells. This localization is due to the constitutive endocytosis of CTLA-4 from the plasma membrane and results in approximately 90% of CTLA-4 being intracellular. One molecular mechanism that is consistent with a dominant cell-extrinsic role for CTLA-4 is the physical capture of CD80 and CD86, and their subsequent removal from APCs: a process known as trans-endocytosis.
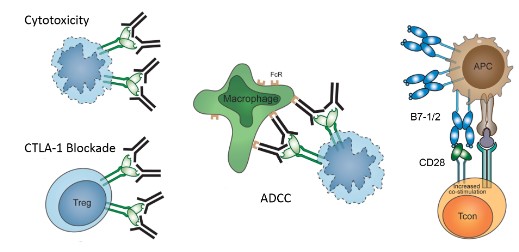
The concept of using immune checkpoint inhibitors to break T cell dysfunction in tumor patients appears to be an intriguing approach to cancer therapy. This was first demonstrated by the success of Ipilimumab, an antiCTLA-4 mAb, resulting in the approval of Ipilimumab by the FDA for advanced melanoma.
PD-1/PD-L1
Initially, PD-1 was demonstrated to be a receptor for cell death; however, the PD-1 pathway was later found to play a regulatory role in inhibiting T cell activation and restraining T cell function. In contrast to CTLA-4, PD-1 predominantly regulates effector T cell activity within tissues and tumors as opposed to regulating T cell activation in lymphoid organs. While CTLA-4 mainly affects naïve T cells, PD-1 is primarily expressed on mature T cells in peripheral tissues and the tumor microenvironment. It is also expressed on other non-T cell subsets including B cells, professional APCs, and natural killer (NK) cells.
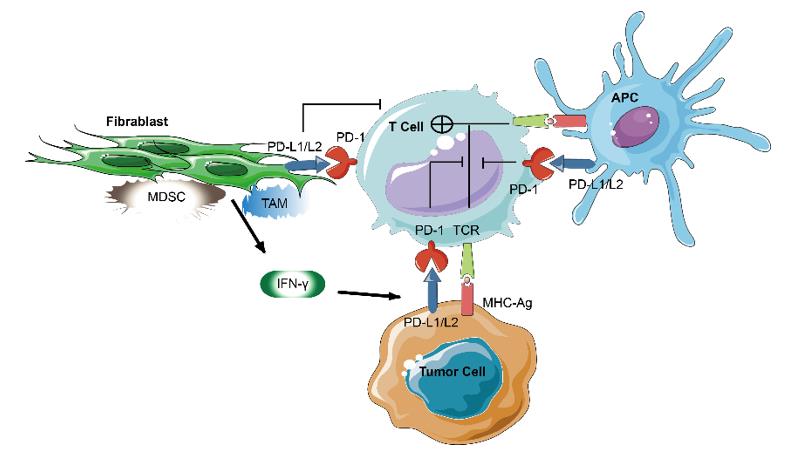
PD-L1 and PD-L2 (also referred to as B7-H1 and B7-DC, respectively) are both type I transmembrane proteins. Though PD-L1 is expressed infrequently in normal human tissues outside of the placenta and macrophage-like cells, its expression is upregulated by IFN-γ and other cytokines that are released by activated T cells. In contrast to the positive costimulatory signal delivered by CD28 receptors, PD-1 delivers a negative signal when bound to its ligands, PD-L1, and PD-L2. PD-1 suppresses T cell activation through the recruitment of phosphatase SHP-2 and the subsequent inactivation of Zap 70, which plays a critical role in T-cell receptor signaling.
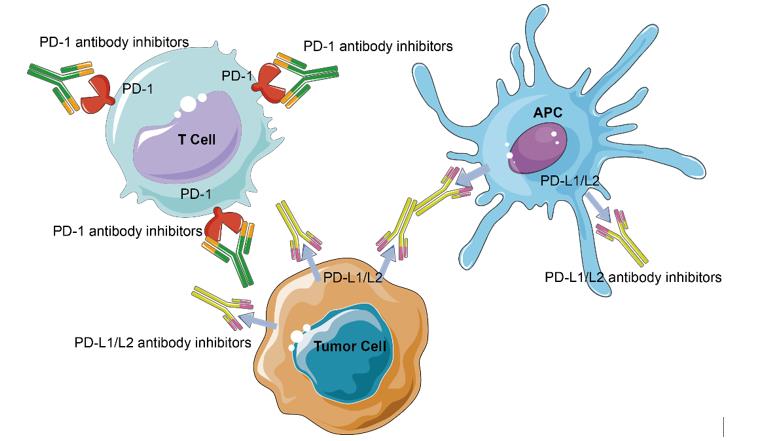
Given the expression of PD-1 on TILs and the upregulation of PD-1 ligands on tumor cells, an anti-tumor host immune response has the foundation. Antibody blockade of PD-1 and its ligands leading to enhanced antitumor immunity in mouse models shows that targeting PD-1 and other immune checkpoints is able to reverse this dysfunctional state and reinvigorate T cell activity in chronic viral infections and cancer.
Immune Checkpoint Therapy
Immune checkpoint blockade is game-changing and revolutionary in at least two senses. First, the target for therapy is on immune cells but not tumor cells. Second, the approach is not to attack tumor-specific antigens but to remove an inhibitory pathway.
Recent studies demonstrated that immune checkpoint inhibitors are able to induce durable, long-lasting cancer control. These inhibitors have shown promising efficacy in melanoma, renal cell carcinoma, NSCLC, and bladder UC. As in the case with immunotherapy for other types of cancers, these drugs show a limited response rate, but the efficacy in achieving long-lasting benefits for some patients has changed the paradigm of cancer treatment.
Some Selected Immunotherapeutic Agents in Clinical Trials
| Target | Name | Antibody Class | Diseases | Status | Company |
|---|---|---|---|---|---|
| CTLA-4 | Lpilimumab (Yervoy) | IgG1k | Melanoma Non-small/small cell lung cancer | FDA Approved | Bristol-Myers Squibb |
| tremelimumab | IgG2 | melanoma | Completed Phase III | Pfizer, now Medimmune | |
| PD-1 | Nivolumab/BMS-936558/MDX1106 | IgG4 | melanoma | Phase III | Bristol-Myers Squibb |
| Pidilizumab/CT-011 | IgG1 | non-Hodgkin's lymphoma, chronic lymphocytic leukemia, Hodgkin's lymphoma, multiple myeloma, acute myeloid leukemia | Phase I/II | CureTech | |
| Pembrolizumab/MK-3475 | IgG4 | HNSCC | Phase I | Merck | |
| PD-L1 | BMS-936559/MDX-1105 | IgG4 | various cancers | Phase I/II | Bristol-Myers Squibb |
| MEDI4736 | IgG1k | Select Advanced Malignancies | Phase I | MedImmune/AstraZeneca |
However, we also need to notice that immunotherapy, helping to prompt an "immune response", can attack healthy cells as well as cancer cells, certain side effects may be experienced, including digestive tract symptoms, loss of appetite, fatigue, and flu-like symptoms.

Quality Guarantee - We Guarantee the Quality of Our Products!
High Purity
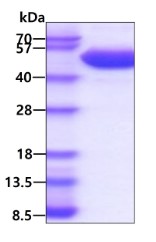
3ug by SDS-PAGE under reducing condition and visualized by coomassie blue stain. The purity of the protein is greater than 95%. (CTLA4-01H)

Human GITR Ligand, Fc Tag on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than
95%. (TNFSF18-37H)
Bioactivity
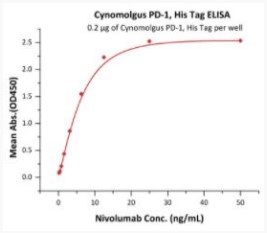
Elisa Test
Immobilized Cynomolgus PD-1, His Tag at 2 μg/mL (100 μL/well) can bind Nivolumab with a linear range of 0.2-13 ng/mL
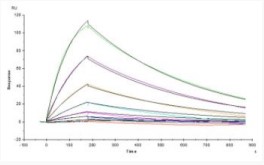
SPR Test
Opdivo (Nivolumab) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Cynomolgus PD-1, His Tag with an affinity constant of 18.1 nM as determined in a SPR assay.
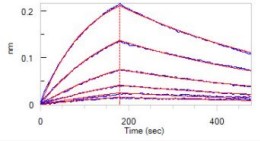
BLI Test
Loaded Opdivo (Nivolumab) on Protein A Biosensor, can bind Cynomolgus PD-1, His Tag with an affinity constant of 17.5 nM as determined in BLI assay.
Case Study
Case 1:
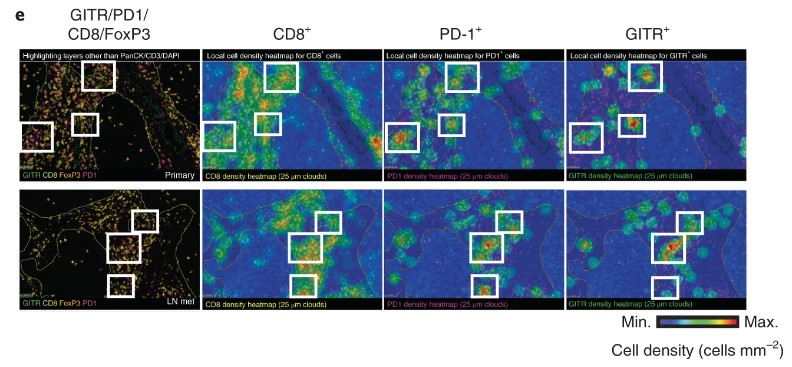
Representative CD8, PD-1(Cat. No. PDCD1-5223C), GITR (Cat. No. TNFRSF18-01C)and FoxP3 multiplex immunofluorescence imaging (excluding PanCK, CD3 and DAPI) and spatial distribution cell density heatmaps (cells mm–2) (CD8, PD-1 and GITR) of FFPE sections of primary HNSCC (excluded compartment) and matching lymph node metastases (LN mets) (20×). Selected areas of GITR+PD-1+CD8+ T cells are indicated in white boxes. Experiment is representative of n = 3 tumor samples.
Case2:
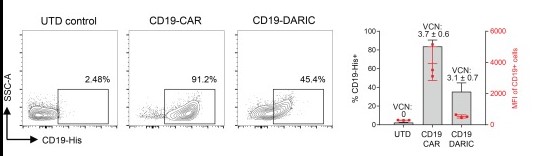
The transduced cells were stained with fluorescently conjugated CD19-His antigen (Cat.No. CD19-3309H) and analyzed by FACS. Summary of 3 donors is shown on the right, with the MFI values in red and the VCN values for each sample annotated above.