The vasculature is the earliest organ to develop. Unlike other organs it has to be functional throughout development and therefore has to constantly adapt to the changing requirements of embryogenesis. As soon as an embryo grows bigger than about 2mm it critically depends on a functional vasculature because passive diffusion is not sufficient to supply all cells with oxygen and nutrients. Similar to embryos, any tumor wanting to grow bigger than 2mm needs functional blood vessels. This will supply the tumor with oxygen and nutrients but also provides a route for the spreading of metastasis. If we find a possible way to prevent tumors vascularized, there may be a cure for cancer. That’s why we are so interested in vascular development.
Blood vessel formation involves vasculogenesis and angiogenesis. Assembly of vascular networks involves sprouting, migration and proliferation of endothelial cells, which is a thin layer of simple squamous cells. Endothelial cells in direct contact with blood are called vascular endothelial cells, whereas those in direct contact with lymph are known as lymphatic endothelial cells.
The mechanism of angiogenesis is still not clear now. There are lots of stimulators involve in the process, such as FGF, VEGF, VEGFR, Ang1, PDGF and so on. Different stimulator has different mechanism.
Vascular endothelial growth factor (VEGF) has been demonstrated to be a major contributor to angiogenesis, increasing the number of capillaries in a given network. Knock out mice which have the gene for VEGF deleted do not develop any vascular endothelial cells at all and die at an early embryonic age.
FGFs are multifunctional proteins with a wide variety of effects; they are most commonly mitogens but also have regulatory, morphological, and endocrine effects. One of the important functions of FGF1 and FGF2 is promotion of endothelial cell proliferation, which may more potent than VEGF or PDGF.
Recent studies have suggested that changes in cellular metabolism are important to these processes. Although much is known about vascular endothelial growth factor (VEGF)-dependent regulation of vascular development and metabolism, little is understood about the role of fibroblast growth factors (FGFs) in this context.
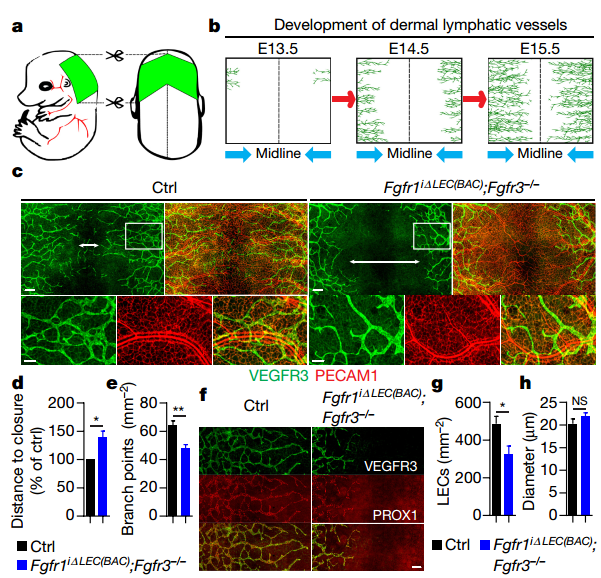
The author generated pan-endothelium (Fgfr1iΔEC;Fgfr3-/-) and lymphatic endothelium (Fgfr1iΔLEC(BAC);Fgfr3-/-) knockout mice. Cre activation at embryonic day (E)12.5 and E13.5
resulted in a high degree of recombination in the skin lymphatic vessels at E15.5 with both Cre deleters.
Fig1. Inhibition of FGF signalling impairs lymphatic development.
Early vascular development is laterally symmetrical (paired left and right). LECs start to invade anterior dorsal skin at E12.5 and migrate towards the dorsal midline. By E15.5–E16, lymphatic vessels from both sides fuse at the dorsal midline forming a primary lymphatic network. Knockout single Fgfr1iΔLEC(BAC) or Fgfr3-/- has no effect on lymphatic front migration. However, LEC-specific Fgfr1/Fgfr3 double knockout mice showed decreased LEC front migration, branching and fewer LECs in the skin. Similarly, arterial vasculature also showed a reduction in branching in Fgfr1/Fgfr3 double knockout mice. Further investigation reveals that knockdown of FGFR1 in human dermal lymphatic endothelial cells (HDLECs) significantly reduced cell proliferation and migration, while FGFR3 downregulation had no effect.
Gene Ontology analysis of LECs after stimulation with FGF2 or FGFR1 knockdown showed enrichment of molecular pathways related to cell proliferation and migration. Besides, there was also enrichment among cellular metabolism processes and, especially, glucose metabolism pathways. FGF signaling activation increased, while FGFR1 downregulation reduced, ATP production in HDLECs, consistent with the major contribution of glycolysis to energy generation.
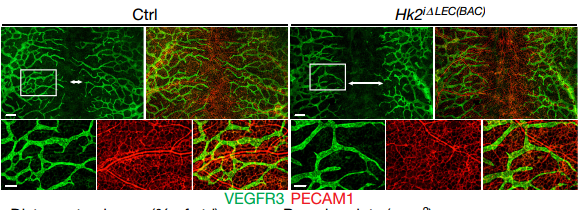
Hexokinase (HK1 and HK2), phosphofructokinase (PFKP) and pyruvate kinase (PKM2) are glycolytic rate-limiting enzymes. HDLEC stimulation with FGF2 induced a robust increase in HK2 expression, with minimal expression changes of other enzymes, while FGFR1 knockdown led to a significant reduction in HK2. RNA-seq data also showed that HK2 was the only glucose metabolic gene among the top 20 transcripts induced by FGF2 and downregulated by FGFR1 knockdown. Further analysis indicated that FGF2 enhanced glycolysis and selectively induced HK2 expression in human umbilical vein endothelial cells (HUVECs), which indicating that FGF regulation of angiogenesis and lymphangiogenesis share similar metabolic mechanisms.
Fig2. HK2 knockout effect lymphangiogenesis.
Cellular biology analysis showed that HK2 knockdown could decrease HDLEC proliferation and migration which stimulated by FGF2, conversely, HK2 overexpression rescued FGFR1 knockdown-induced decrease. HK2 knockout mice embryos displayed a reduction in the extent of lymphatic vessel branching and migration towards the midline. Besides, there were a higher proportion of G1 and smaller proportion of S-phase cells in Hk2-deficient LECs.
The author further examine the role played by Hk2 in adult lymphangiogenesis. FGF2-containing pellets were implanted into HK2 knockout mice, which was activated at the adult stage. FGF2 pellet implantation led to robust stimulation of lymphangiogenesis in control mice that was significantly reduced in Hk2iΔLEC(KI) mice.
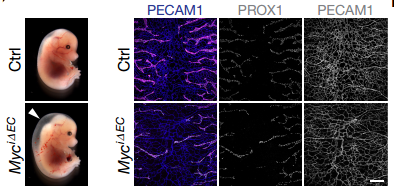
Previous observation showed that MYC binding to the regulatory region of the HK2 gene, the author further examined whether MYC links FGF signaling to HK2 transcription in HDLECs. The result revealed that HK2 mRNA level changed consistence with MYC level, and MYC knockdown also reduced glycolysis, while overexpression increased glycolytic activity. Additionally, FGF2 could also increase MYC protein. Furthermore, ChIP–quantitative PCR (ChIP–qPCR) showed that the amount of MYC binding to the HK2 E-boxes was increased by FGF2 treatment and reduced by FGFR1 knockdown. Other cellular and mice model analysis concluded that FGF-dependent regulation of MYC expression underlies control of HK2 levels in LECs and BECs.
Fig3. MYC knockout effect lymphatic development.
All experimental data concluded that FGF signaling plays a pivotal role in both blood and lymphatic vascular development and it is also required for lymphangiogenesis in tumors. At the molecular level, FGFs control glycolysis through an MYC-dependent regulation of HK2 expression. FGF stimulation increased HK2 levels leading to induction of glycolysis and increased production of glycolytic metabolites, while its suppression had the opposite effect. MYC mediates FGF2 effects on HK2 expression by directly binding to HK2 regulatory elements and controlling its transcription.
Given the mice which with tumors FGFR inhibitor orally could reduce lymphangenesis in the peri-tumoural area, indicating a potential therapeutic value of FGFR inhibitors as anti-lymphangiogenic agents. Therapeutic targeting of this FGF–MYC–HK2 pathway may open new possibilities for treatment of diseases associated with insufficient or excessive vascular growth.
Reference
1. Pengchun Yu et al. FGF-dependent metabolic control of vascular development. Nature. 2017 May 3. doi: 10.1038/nature22322.