The development of normal mammals occurs in a hydrogen-peroxide environment, and the adaptive response to hydrogen peroxide is considered a landmark event for embryo survival in hypoxic fallopian tubes and intrauterine environments, thus playing a crucial role in mammalian evolution. Due to the gene deletion of hypoxia-inducible factor (HIF) (oxygen-sensitive transcription factor), the response to physO2 is insufficient, resulting in embryo loss during implantation or severe developmental defects shortly after implantation. However, due to significant morphological defects occurring mainly during the implantation and post-implantation stages, the developmental role of HIF in supporting pre-implantation development has long been overlooked.
Consistent with insufficient response to physO2 leading to developmental failure, strong epidemiological evidence suggests that atomosO2 during human in vitro fertilization (IVF) culture is typically associated with lower clinical pregnancy and live birth rates. In addition, a large number of animal studies have consistently shown that compared to low oxygen concentration in vitro culture, in vitro culture in atomosO2 can damage the morphological parameters of pre-implantation embryos and subsequent pregnancy outcomes.
These facts indicate that short-term exposure to the atmosphere can irreversibly damage the developmental potential of pre-implantation embryos, leading to unsatisfactory pregnancy outcomes after embryo transfer. There are some reasonable explanations, such as exposure to the atmosphere leading to increased levels of reactive oxygen species (ROS) in pre-implantation embryos, increased incidence of DNA breakage and apoptosis, metabolic-related gene dysregulation, and changes in mitochondrial structure and function. However, the underlying mechanism by which atmospheric sulfur dioxide damages embryonic potential, especially during the cleavage stage, remains largely unknown.
Our Featured Products
| Cat.No. | Product Name | Source | Species | Tag |
| HIF1A-106H | Recombinant Human HIF1A, GST-tagged | Wheat Germ | Human | GST |
| HIF1A-107H | Recombinant Human HIF1A, His-tagged | E.coli | Human | His |
| HIF1A-3092H | Recombinant Human HIF1A, His tagged | E.coli | Human | His |
| HIF1A-145H | Recombinant Human HIF1A protein, His-tagged | E.coli | Human | His |
| HIF1A-198H | Recombinant Human HIF1A protein, GST-tagged | E.coli | Human | GST |
Recently, researchers from China Agricultural University published an article in Redox Biology titled “Single-embryo transcriptomic atlas of oxygen response reveals the critical role of HIF-1α in prompting embryonic zygotic genome activation”. This study revealed the key role of HIF-1α in promoting embryonic zygotic genome activation through a single embryo oxygen response transcriptome profile.
The adaptive response to physiological oxygen levels (physO2; 5% O2) enables embryos to survive in a hypoxic developmental environment. However, the mechanism by which physO2 supports pre-implantation development is still unclear. In this study, the researchers systematically investigated the oxygen response of landmark events during pre-implantation development.
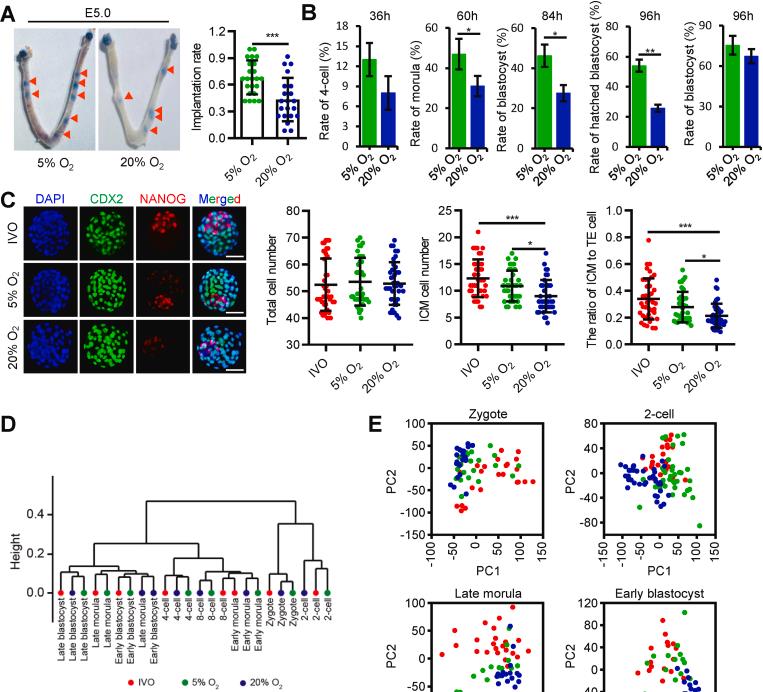
Focusing on atmospheric oxygen levels (atomosO2; 20% O2), researchers have functionally identified HIF-1α as an oxygen-sensitive transcription factor in promoting major zygote genome activation. In addition, during the formation of blastocysts, atmospheric sulfur dioxide inhibits the deposition of H3K4me3 and H3K27me3 by regulating histone lysine methyltransferase, thereby damaging the inactivation of the X chromosome in the blastocyst.
In addition, researchers have found that before blastocyst formation, atmospheric sulfur dioxide hinders the transition from metabolism to glycolysis, leading to low-level deposition of histone lactate. It is worth noting that researchers have also reported an increase in the biphasic oxygen response during embryo development before implantation.
In summary, this study focuses on the genetic and epigenetic events necessary for embryonic survival and development, advancing existing knowledge of the adaptive response of embryos to physO2, and providing new insights into the mechanism of irreversible developmental potential impairment caused by short-term exposure to SO2 in the atmosphere.
By integrating single embryo transcriptome, phenotype quantification, and functional analysis, researchers systematically investigated the effects of oxygen concentration on landmark developmental events, including zygote genome activation, lineage differentiation, metabolic transfer, and related genetic or epigenetic characteristics. The current research advances the existing knowledge of the adaptive response of embryos to physO2 survival in physiological hypoxic environments and provides new insights into the mechanisms of irreversible developmental potential damage caused by short-term exposure to the atmosphere.
In summary, this study provides a new understanding of the impact of oxygen concentration on pre-bed genetic or epigenetic programming and reprogramming events. These results not only update the current understanding of the functional role of physO2 in promoting embryo survival and development, but also provide a deeper understanding of the beneficial effects of low oxygen concentrations on pregnancy outcomes in clinical practice of assisted reproductive technology.
Related Products and Services
Embryonic and Induced Pluripotent Stem Cells
Protein Expression and Purification Services
Reference
Fusheng Yao et al. Single-embryo transcriptomic atlas of oxygen response reveals the critical role of HIF-1α in prompting embryonic zygotic genome activation. Redox Biol. 2024 Apr 2:72:103147. doi: 10.1016/j.redox.2024.103147.