The occurrence of many childhood cancers often begins with molecular hijacking. Recently, a research report titled “Heterotypic interactions can drive selective co-condensation of prion-like low-complexity domains of FET proteins and mammalian SWI/SNF complex” was published in the journal Nature Communications. Scientists from institutions such as the University of Buffalo revealed through their research how fusion proteins intercept gene regulators to stimulate the development of cancer in children.
In the article, researchers found that a group of abnormal proteins called fusion proteins can abnormally bind to a group of proteins that turn on and off gene expression. Therefore, genes that should have been activated are suppressed, while genes that should have been suppressed are activated, leading to cancer.
In this study, researchers have gained new insights into the molecular mechanisms behind this hijacking. They found that fusion proteins and a gene regulatory protein complex can interact through their unopened and loose regions, known as disordered domains. Although it has fuzzy properties, this disordered structural domain can have high specificity by forming liquid-like droplets and mixing them evenly.
Researcher Priya Banerjee said that there is ample research evidence to suggest that disordered domains of proteins may play a crucial role in the development of cancer and other major human diseases, but the problem lies in how they function. In this study, researchers found that key network events between fusion proteins and gene regulatory protein complexes can occur through this disordered domain, and this process has a certain chemical specificity.
Fusion proteins are the result of a chromosome breaking and attaching to another chromosome to form fusion genes. Fusion proteins do not always cause cancer, but some can alter the activity of other genes, causing uncontrolled cell growth and ultimately leading to cancer. These are all called fusion oncoproteins.
Our Featured Products
| Cat. No. | Product Name | Source (Host) | Species | Tag |
| SMARCA4-2804H | Recombinant Human SMARCA4, GST-tagged | E.coli | Human | GST |
| SMARCA4-198H | Recombinant Human SMARCA4 Protein, His-tagged | E.coli | Human | His |
| SMARCA4-01H | Recombinant Human SMARCA4 Protein, FLAG-tagged | Insect Cells | Human | FLAG |
| SMARCA2-2657H | Recombinant Human SMARCA2 protein, His-tagged | E.coli | Human | His |
| SMARCB1-3763H | Recombinant Human SMARCB1 protein, GST-tagged | E.coli | Human | GST |
| SMARCD2-5273R | Recombinant Rat SMARCD2 Protein, His (Fc)-Avi-tagged | HEK293 | Rat | His (Fc)-Avi |
| SMARCA1-1538HFL | Recombinant Full Length Human SMARCA1 Protein, C-Flag-tagged | Mammalian cells | Human | Flag
|
| SMARCA1-1919H | Recombinant Human SMARCA1 Protein, MYC/DDK-tagged | HEK293 | Human | Myc/DDK |
To promote the development of cancer, many mistakes need to be made, which means that a large number of genetic mutations need to occur. However, in some cases of childhood cancer, these fusion oncoproteins may be the only culprit. Therefore, targeting oncoproteins alone may be sufficient to cure such childhood cancer. Other researchers have found through research that fusion oncoproteins can alter gene activity by intercepting major chromatin remodeling proteins called mammalian SWI/SNF complexes. Approximately 20% of all human cancers carry mutations in this protein complex.
So researchers Banerjee et al. wanted to know how this fusion oncoprotein hijacked this complex. This latest study is based on a study published by researchers in 2021, which found that fusion oncoproteins and mammalian SWI/SNF complexes can interact through their respective disordered domains. Due to folding to form a three-dimensional shape, disordered domains have long been a mystery for scientists. The scientific dogma for decades is that interactions between proteins occur through lock and key-like mechanisms in the structural regions of proteins.
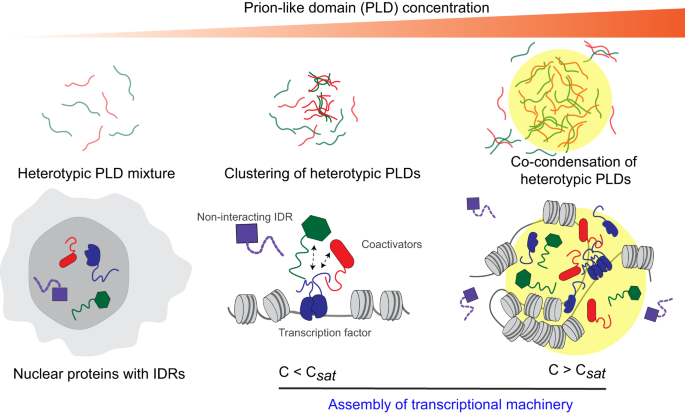
However, research results from the past two decades have shown that nearly 30% of human proteomes are disrupted, and these disrupted regions drive key biological processes and the occurrence of major human diseases. Now researchers have observed special disordered domains and gene regulatory complexes called prion-like domains from fusion oncoproteins, and found that these special domains can form liquid-like droplets or membrane-less organelles in the nucleus.
When the network of interactions between oncoproteins and gene regulators begins, their prion-like domains form molecular complexes, which then bind and form liquid-like droplets or co-condensates. To further investigate, researchers used a liquid activated by blue light for systematic research, which can help researchers observe these processes more clearly. However, there may be subtle differences in what can cause co-aggregation and activate abnormal gene expression to induce cancer.
Researcher Banerjee stated that we found that the inhibitory of proteins’ disordered domains do not truly accumulate in droplets formed by prion-like domains. We believe that this selectivity in the molecular network is an important mechanism for fusing oncoproteins to promote cancer development. Once the fusion protein successfully intercepts the complex, it can essentially take it away from the genes it should open and bring it to the genes it should not open. Future researchers still hope to develop new pharmacological drugs to target abnormal networks of fusion proteins carrying SWI/SNF complexes, thereby curing cancer.
Finally, the researchers state that this fundamental study is very important, and unless researchers fully understand the molecular mechanisms hidden behind deadly human diseases, new therapies cannot be developed to treat them.
Related Products and Services
| SMARCA4 | SMARCB1 | SMARCA5 | SMARCD3 |
| SMARCA2 | SMARCD2 | SMARCA1 | SMARCE1 |
Protein Expression and Purification Services
Reference
Davis, R.B., Supakar, A., Ranganath, A.K. et al. Heterotypic interactions can drive selective co-condensation of prion-like low-complexity domains of FET proteins and mammalian SWI/SNF complex. Nat Commun 15, 1168 (2024). doi:10.1038/s41467-024-44945-5